MEETING HIGHLIGHT
Zolbetuximab: The role of CLDN18.2-targeted therapies in treating HER2-negative advanced gastric cancer
Gastric cancer and gastroesophageal junction cancer (GC/GEJC) continue to pose major global health challenges.1-4 Claudin18.2 (CLDN18.2), a tight junction protein normally restricted to gastric mucosa, is increasingly recognized as a promising therapeutic target, as it becomes aberrantly expressed and accessible in malignant gastric cells.5,6 At a recent symposium, Professor Florian Lordick from the University of Leipzig, Germany, discussed the biology of CLDN18.2 and its clinical relevance in targeting human epidermal growth factor receptor 2 (HER2)-negative advanced gastric adenocarcinoma with zolbetuximab—highlighting its potential as a first-line treatment.
CLDN18.2 as a novel therapeutic target in HER2-negative gastric adenocarcinoma
GC/GEJC ranked 5th in incidence and 4th in cancer-related deaths globally in 2020, with over 1 million new cases and approximately 769,000 deaths reported.1 According to European Society for Medical Oncology (ESMO) guidelines, the current standard first-line therapy involves fluoropyrimidines and platinum-based chemotherapy.2 However, new treatment targets are urgently needed to expand options for patients with advanced disease.3,4
CLDN18.2 is a tight junction protein typically confined to the gastric mucosa. However, during malignant transformation, these junctions are disrupted, exposing CLDN18.2 on tumor cell surfaces and making it an ideal target for therapies.4-6
Prof. Lordick further emphasized that CLDN18.2 forms tight junctions between gastric mucosa cells via apical polarity.5 During carcinogenesis, the disruption of this polarity leads to the loss of tight junctions, exposing CLDN18.2 on the tumor cell surface, making it more accessible for targeted therapies.5
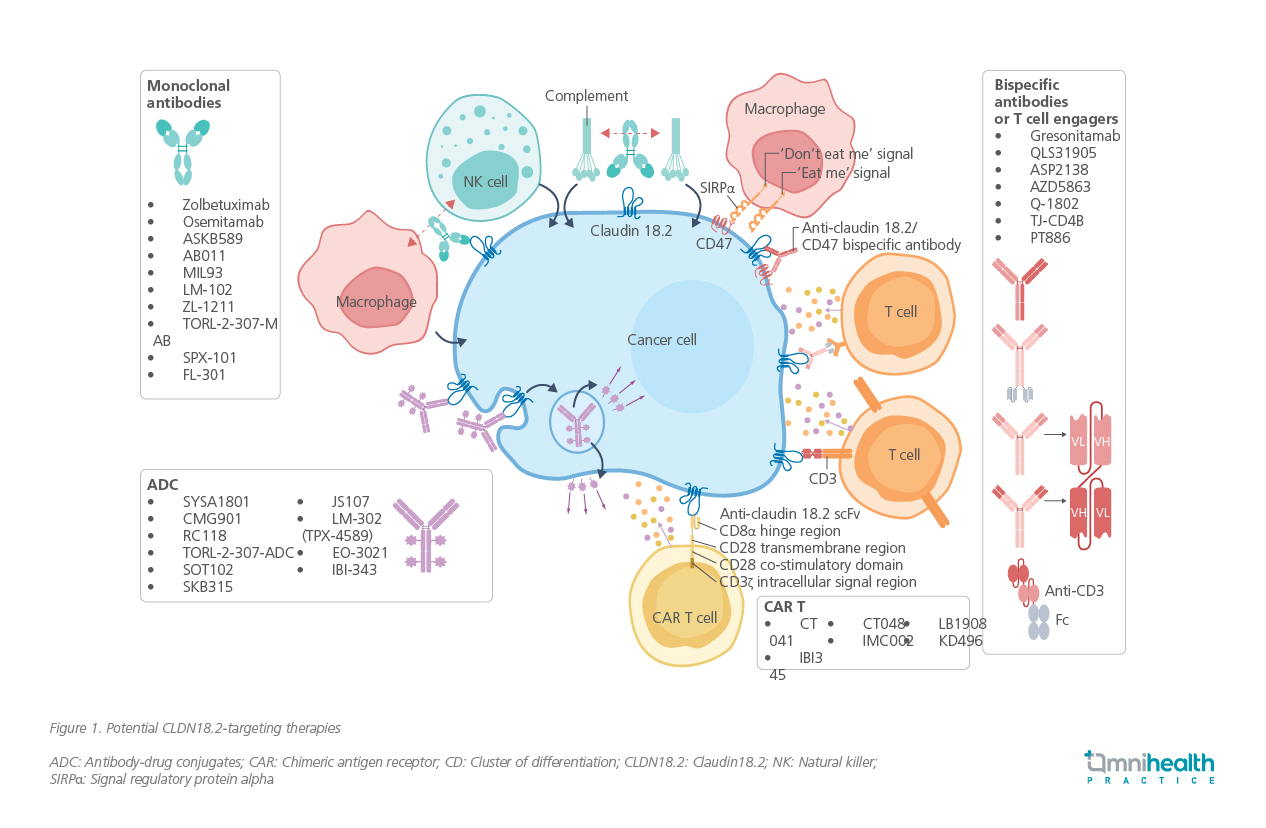
The prevalence of CLDN18.2 expression in GC/GEJC is well documented.6 In a study involving 65 patients with advanced disease, 73.8% (n=48) tested positive for CLDN18.2.6 Moreover, a pooled analysis of two phase 3 trials involving 4,507 patients found that 38.4% had CLDN18.2-positive tumors further supporting its relevance as a treatment target.7 This growing evidence has spurred the development of multiple CLDN18.2-targeted agents (figure 1).5
Early efficacy of zolbetuximab demonstrated in the phase 2 FAST trial
Zolbetuximab is a first-in-class monoclonal antibody that binds specifically to CLDN18.2, inducing tumor cell death via antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).4
Although zolbetuximab monotherapy showed limited efficacy in the MONO study, combining it with chemotherapy has demonstrated clear advantages.8-11 This approach enhances T-cell infiltration and stimulates the release of pro-inflammatory cytokines, leading to improved clinical outcomes and better survival benefits for patients with advanced GC/GEJC (table 1).4,9
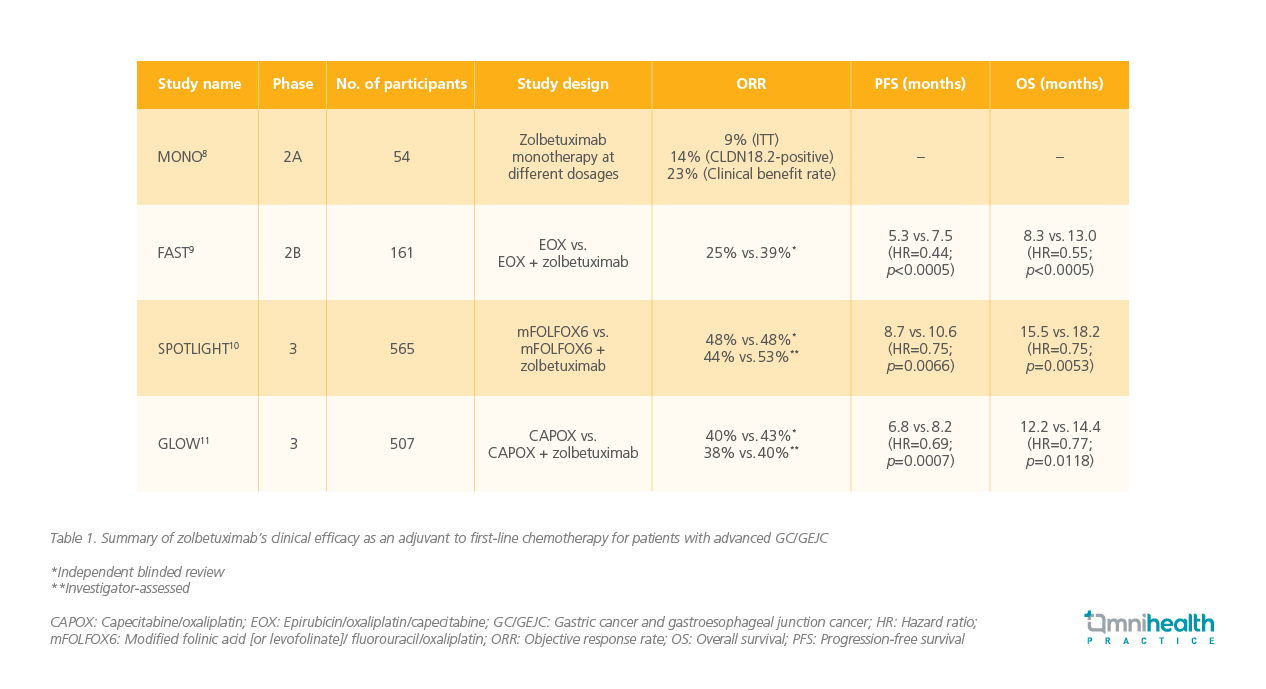
The clinical efficacy of zolbetuximab was initially demonstrated in the FAST trial, a randomized, multicenter phase 2 study that compared the effectiveness and tolerability of zolbetuximab in combination with epirubicin/oxaliplatin/capecitabine (EOX) against EOX alone as the first-line treatment for advanced GC/GEJ patients expressing CLDN18.2.9 In the study, 161 adult patients who tested positive for CLDN18.2 (defined as ≥40% of tumor cells with 2+ or 3+ staining intensity on CLAUDETECT 18.2 immunohistochemistry assay) were randomly assigned to receive either EOX alone (n=84) every 3 weeks or zolbetuximab + EOX (loading dose of 800mg/m2, followed by 600mg/m2 every 3 weeks) (n=77) for 8 cycles (21 days/cycle).9 The zolbetuximab + EOX group showed significantly longer median progression-free survival (mPFS) (7.5 months vs. 5.3 months; HR=0.44; 95% CI: 0.29-0.67; p<0.0005) and overall survival (OS) (13.0 months vs. 8.3 months; HR=0.55; 95% CI: 0.39-0.77; p<0.0005) compared to the EOX alone group.9
“A very important finding from this study is that a higher expression level of CLDN18.2 was required to achieve this effect,” Prof. Lordick remarked. Specifically, the survival benefits brought by zolbetuximab were more pronounced in patients with ≥70% of tumor cells positive for CLDN18.2, demonstrating statistical significance for both PFS and overall survival OS (p<0.0005).9 Conversely, there was no statistically significant difference in median PFS and OS between the two cohorts for patients with 40%-69% of tumor cells positive for CLDN18.2 (p=0.497 for PFS, and p=0.401 for OS).9 Regarding safety, fewer serious adverse events (AEs) (24.7% vs. 32.1%) and fatal AEs (10.4% vs. 17.9%) occurred in the zolbetuximab + EOX group.9
SPOTLIGHT and GLOW demonstrate efficacy of zolbetuximab in advanced GC/GEJC
The SPOTLIGHT and GLOW phase 3 trials provided further validation of zolbetuximab’s efficacy in improving survival outcomes for patients with advanced GC/GEJC.10,11 The SPOTLIGHT study enrolled 565 adult patients with CLDN18.2-positive (defined as ≥75% of tumor cells showing moderate-to-strong membranous CLDN18 staining), HER2-negative (based on local or central evaluation), previously untreated, locally advanced unresectable, or metastatic GC/GEJC.10 Patients were randomized to receive either modified folinic acid [or levofolinate]/fluorouracil/oxaliplatin regimen (mFOLFOX6) + placebo or mFOLFOX6 + zolbetuximab (loading dose of 800mg/m2, followed by 600 mg/m2 every 3 weeks).10 At the end of the study, zolbetuximab was associated with a significantly longer mPFS (10.61 months vs. 8.67 months; HR=0.75; 95% CI: 0.60-0.94; p=0.0066) and OS (18.23 months vs. 15.54 months; HR=0.75; 95% CI: 0.60-0.94; p=0.0053) compared to placebo.10 Prof. Lordick emphasized that the OS benefits induced by zolbetuximab were more pronounced in Asian patients compared to Caucasian patients (HR for Asian=0.57 [95% CI: 0.39-0.83], HR for Caucasian=0.95 [95% CI: 0.70-1.29]).10 Similarly, the GLOW trial enrolled 507 adult patients with previously untreated, locally advanced unresectable, or metastatic gastric or gastroesophageal junction (G/GEJ) adenocarcinomas.11 Patients were randomly assigned to receive either capecitabine/oxaliplatin (CAPOX) + placebo (n=253) or CAPOX + zolbetuximab (n=254).11 Patients treated with zolbetuximab group experienced longer mPFS (8.21 months vs. 6.80 months; HR=0.687; 95% CI: 0.544-0.866; p=0.0007) and OS (14.39 months vs. 12.16 months; HR=0.771; 95% CI: 0.615-0.965; p=0.0118) compared to those in the placebo group.11
Shaping the future landscape of gastric adenocarcinoma treatment
Zolbetuximab’s consistent and reliable clinical benefits in patients with CLDN18.2-positive advanced GC/GEJC led to the proposal of its addition as a first-line treatment option in international clinical guidelines, presenting as the first-in-class CLDN18.2-targeting therapy (figure 2).2,4 Prof. Lordick concluded that the development of zolbetuximab and the validation of CLDN18.2 as a potential cancer target have paved the way for the advancement of other targeted therapies.3-5 Looking ahead, the ILUSTRO multicohort trial is investigating zolbetuximab alone or in combination with immunotherapies in later-line settings—offering further promise for expanding its therapeutic use.12
Conclusion
The identification of CLDN18.2 as a viable therapeutic target has transformed the treatment paradigm for advanced GC/GEJC.4,5 Zolbetuximab, a monoclonal antibody targeting CLDN18.2, has shown remarkable efficacy in improving survival outcomes when used in combination with standard chemotherapy regimens.9-11 As the first-in-class agent in this space, zolbetuximab represents a major step forward in targeted therapy, with growing evidence supporting its integration into clinical practice and guidelines.2,4

