CASE REVIEW
Case sharing: Tezepelumab targets upstream inflammation to bridge the divide in both severe T2-high and -low asthma
Asthma is a complex, chronic respiratory disease characterized by heterogeneous airway inflammation and variable clinical presentation.1 Within its spectrum, severe asthma represents a high-burden subgroup that contributes disproportionately to morbidity, healthcare utilization, and mortality.1,2 Despite advances in biologic therapies targeting type 2 (T2) inflammation, many patients remain inadequately controlled, particularly those with nonallergic or noneosinophilic phenotypes.2 Tezepelumab, a first-in-class monoclonal antibody targeting thymic stromal lymphopoietin (TSLP), acts upstream in the inflammatory cascade to modulate multiple immune pathways, offering broad efficacy across both T2-high and T2-low asthma phenotypes.3,4 In an interview with Omnihealth Practice, Dr. Philip H Li and his team shared their clinical experience with tezepelumab, illustrating its real-world impact in patients at both ends of the T2 spectrum.
Background
Severe asthma remains a clinically challenging condition, affecting approximately 5%-10% of patients with asthma, yet accounting for disproportionate morbidity, healthcare utilization, and loss of quality of life.1,2,4 Many patients with severe asthma receive prolonged courses of oral corticosteroids (OCS) which can incur long-term systemic adverse effects.1,3 Biologics offer a new mode of therapy for these patients to reduce their OCS reliance.1 Traditional biologic therapies target downstream type 2 (T2) inflammatory pathways, such as interleukin (IL)-5 or IL-4/13, providing benefit primarily to patients with T2-high phenotypes.3 However, a substantial subset of patients—particularly those with low eosinophil counts or low fractional exhaled nitric oxide (FeNO) derive limited benefit from these agents, leaving an ongoing unmet clinical need.2,4
Tezepelumab is a first-in-class human monoclonal antibody that inhibits thymic stromal lymphopoietin (TSLP), an epithelial-derived cytokine that orchestrates the initiation and amplification of airway inflammation across multiple pathways, including both T2-high and T2-low cascades.2,3 By targeting an upstream driver of inflammatory mediators, tezepelumab modulates immune and structural cell signaling, reducing airway hyperresponsiveness, and mucus production.2,3 Its unique mechanism allows for broad efficacy across diverse asthma phenotypes, independent of biomarker status.3 Tezepelumab's efficacy and safety have been demonstrated through clinical trials, including the PATHWAY trial and the pivotal NAVIGATOR trial, which showed significant reductions in asthma exacerbation rates and improvements in lung function across diverse T2 asthma phenotypes.2,5 Real-world evidence further reinforced the effectiveness of tezepelumab in routine clinical practice.1
Case sharing
To illustrate how the clinical findings translate into local practice, Dr. Li and his team shared two representative cases—a T2-high and a T2-low phenotype—that underscore tezepelumab’s role in bridging inflammatory subtypes in severe asthma management.
Case 1: Severe T2-high asthma
A 52-year-old woman with severe aspirin-exacerbated respiratory disease (AERD) and a history of recurrent intensive care unit (ICU) admissions presented with poorly controlled asthma despite maximal conventional therapy, including an inhaled corticosteroid/long-acting β₂-agonist/long-acting muscarinic antagonist (ICS/LABA/LAMA) regimen.
In December 2024, physical examination revealed severe airflow limitation (pre-bronchodilator: forced expiratory volume in one second and forced vital capacity (FEV1/FVC) ratio 31%; peak expiratory flow (PEF) 1.56L/sec) and an Asthma Control Test (ACT) score of 10. Given her T2-high inflammatory profile, and restricted access to other biologics due to financial difficulty, she was initiated on a trial of tezepelumab. She was trained on self-administration and tolerated the first dose without adverse events.
A 6-month follow-up revealed a remarkable response. The patient was exacerbation-free, and her ACT score improved to 19, indicating well-controlled asthma. Lung function also improved dramatically, with her pre-bronchodilator FEV1/FVC ratio increasing to 46% and her peak expiratory flow to 2.18L/sec, respectively. This case demonstrates the profound efficacy of tezepelumab in improving both symptom control and lung function in a patient with severe, refractory T2-high asthma.
Case 2: Severe T2-low asthma
A 31-year-old man with a 17-year history of severe, OCS-dependent asthma (prednisolone 60mg/day) was referred for specialist evaluation. He had experienced frequent hospitalizations and OCS-related complications, including osteoporosis, cushingoid features, and secondary immunodeficiency managed with intravenous immunoglobulin (IVIg). Phenotyping—including immunoglobulin E (IgE) levels and other inflammatory markers, failed to identify a clear allergic or inflammatory trigger, consistent with T2-low asthma.
In view of his T2-low phenotype and poor control despite maximal therapy, he had undergone two rounds of bronchial thermoplasty without sustained benefit. He was subsequently initiated on tezepelumab. Within two weeks, he reported noticeable improvement in symptoms and quality of life. His daily prednisolone dose was successfully tapered from 60mg to 15mg, with the goal of complete cessation. This case highlights tezepelumab's efficacy in improving clinical outcomes and reducing OCS burden in severe, T2-low asthma.
Breaking the cycle of asthma exacerbations
Both cases presented exemplify tezepelumab’s ability to interrupt recurrent exacerbation cycles that perpetuate airway decline in severe asthma, which aligns with findings from the randomized controlled trials. The phase 2b PATHWAY trial (n=550; adults aged 18-75 years) established the dose-dependent efficacy of tezepelumab over 52 weeks in severe, uncontrolled asthma.2,5
In the pivotal NAVIGATOR trial, tezepelumab reduced the annualized asthma exacerbation rate (AAER) by 56% compared with placebo (rate ratio [RR]=0.44; 95% CI: 0.37-0.53; p<0.001), with consistent benefits across blood eosinophil and FeNO subgroups (figure 1).2
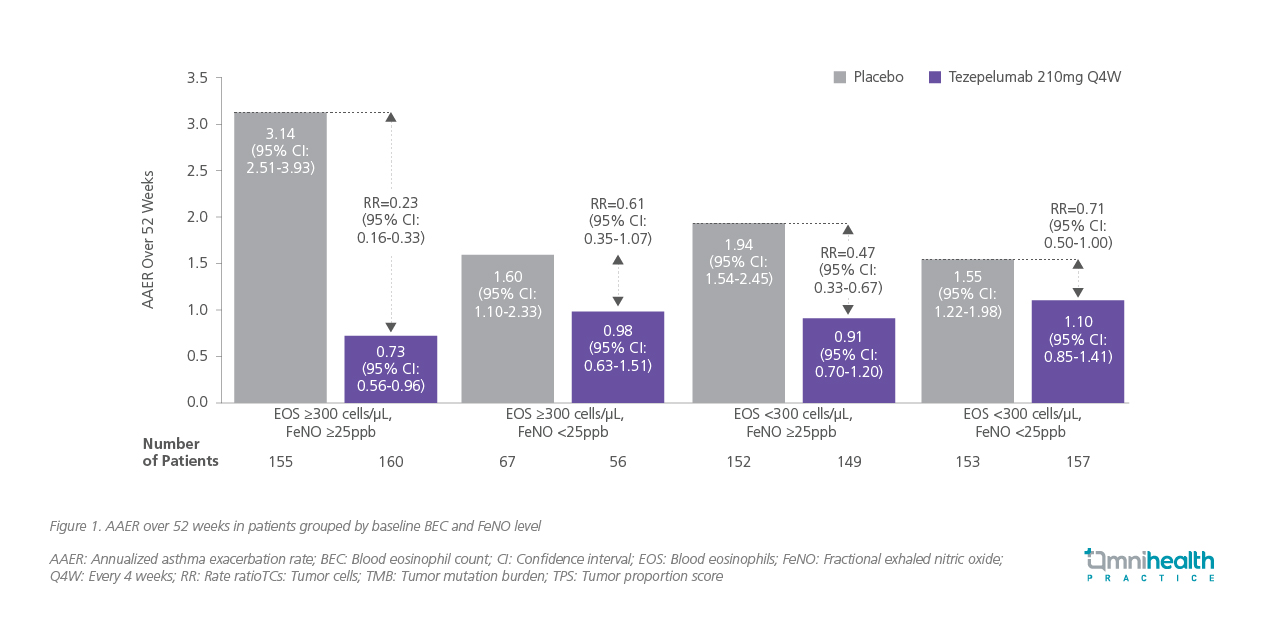
Pooled analyses of NAVIGATOR and PATHWAY reinforced these findings, demonstrating durable reductions in exacerbations across subgroups stratified by allergy status, baseline corticosteroid use, and exacerbation history.4 Tezepelumab achieved a 48% reduction in exacerbation rate among patients with blood eosinophil counts <150 cells/μL and a 48% reduction among those <300 cells/μL —magnitudes of improvement not previously observed with other biologics targeting downstream T2 pathways (figure 2).2,4 This broad efficacy is reflected in Case 2, where a patient with T2-low asthma achieved rapid and marked improvements in symptoms and lung function following tezepelumab initiation.
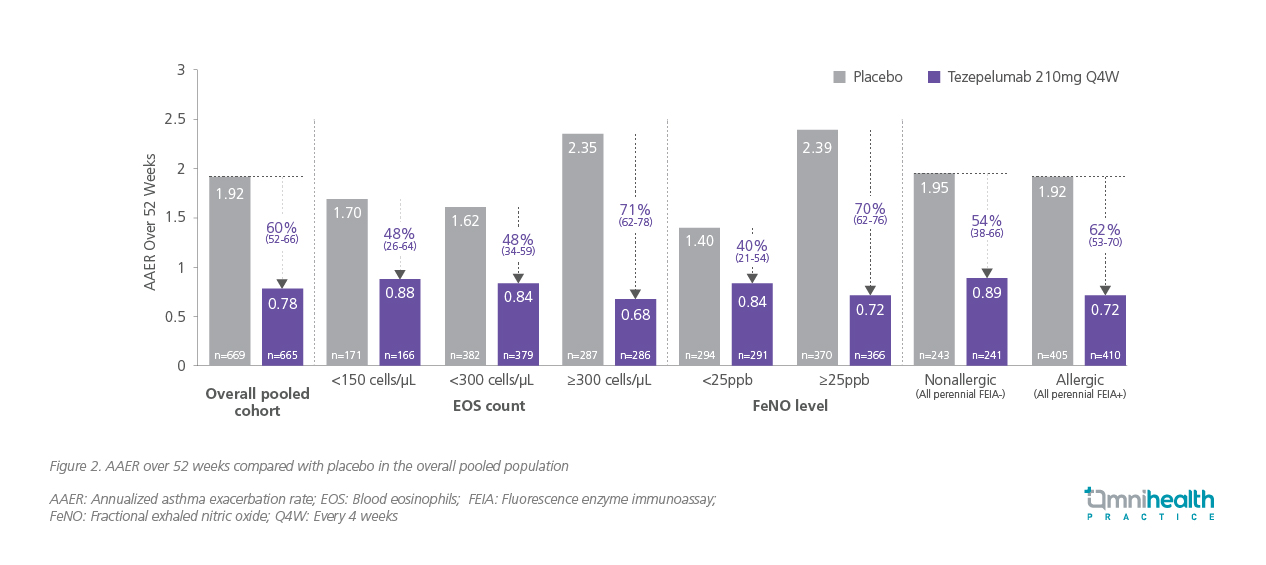
Real-world evidence from multicenter adult cohorts treated with tezepelumab (n=103) complemented these results.1 Exacerbation rates, lung function, and corticosteroid use over one year of followup were assessed.1 Notably, 39% of patients had prior biologic exposure, and 32% had baseline eosinophil counts <300 cells/μL, allowing evaluation across both T2-high and T2-low phenotypes.1 At 1-year follow-up, there was a 66.7% relative reduction in annual exacerbations compared to baseline, with comparable benefits across biomarker-defined subgroups.1 Collectively, these data demonstrate that tezepelumab can effectively mitigate exacerbation risk irrespective of inflammatory phenotype.1
Restoring lung function across phenotypes
Beyond exacerbation prevention, both cases also demonstrated measurable improvements in lung function, echoing the results observed in clinical trials. Pooled analyses from NAVIGATOR and PATHWAY found consistent improvements, with a least-squares mean difference of 0.13L (95% CI: 0.09-0.18) favoring tezepelumab.4 These effects were evident across T2-high and T2-low subgroups, underscoring the mechanistic advantage of upstream TSLP inhibition in improving airway function irrespective of biomarker profile.3,4
Real-world data further supported these findings, demonstrating significant overall improvements in lung function after approximately one year of tezepelumab treatment.1 Median FEV1 % predicted increased from 65% to 74%, and absolute FEV1 improved from 1.69L to 1.90L (both p<0.01).1 Directional improvements were observed across all patient subgroups, including those with prior biologic exposure and across eosinophil strata (<300 and ≥300 cells/μL), with the largest numerical gains noted among biologic-naïve patients with higher baseline eosinophil counts (figure 3).1
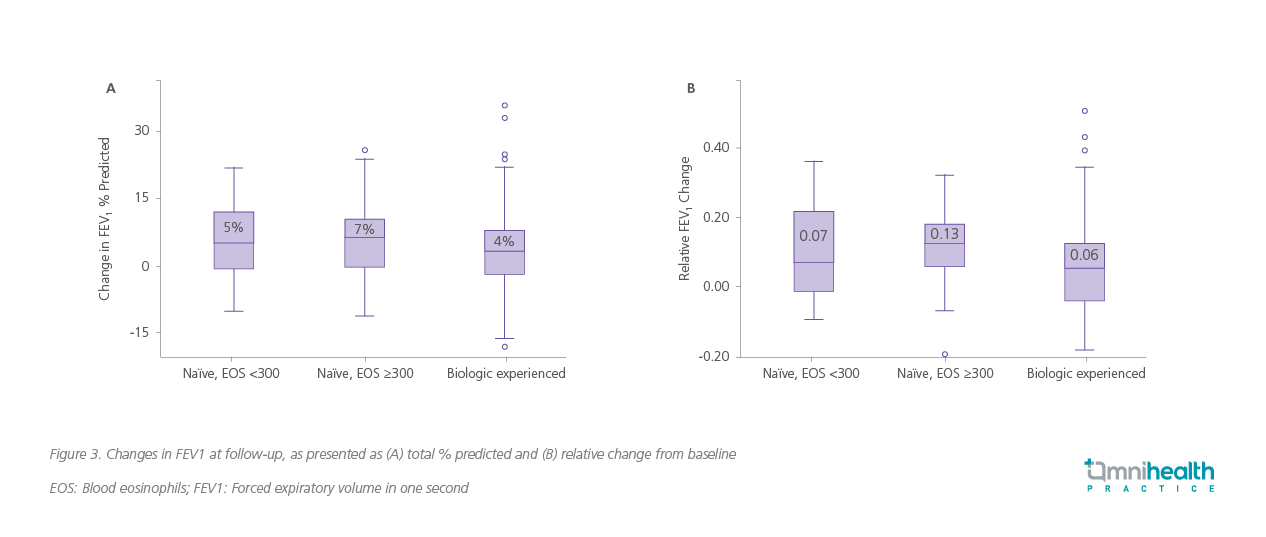
Clinically meaningful symptom improvement and OCS reduction
Improving symptom burden and minimizing corticosteroid dependence remain key treatment goals for patients with severe asthma.1 Patient-reported outcomes (PROs) such as the Asthma Control Questionnaire-6 (ACQ-6), Asthma Quality of Life Questionnaire (AQLQ), and Asthma Symptom Diary (ASD) provide valuable measures of these benefits.2
In NAVIGATOR, tezepelumab demonstrated statistically and clinically significant improvements across all PROs vs. placebo: ACQ-6 (−1.55 vs. −1.22; difference=−0.33; 95% CI: −0.46 to −0.20; p<0.001), AQLQ (1.49 vs. 1.15; difference=0.34; 95% CI: 0.20-0.47; p<0.001), and ASD (−0.71 vs. −0.59; difference= −0.12; 95% CI: −0.19 to −0.04; p=0.002).2 In real-world cohorts, up to 45% of patients discontinued and 23% reduced OCS dose by ≥50%, highlighting that clinical benefit can extend beyond symptom control to corticosteroid minimization in appropriately selected patients.1
Safety and tolerability profile
Across pivotal and pooled studies, tezepelumab demonstrated a favorable safety and tolerability profile.3,4 The most common adverse events (≥5%) were nasopharyngitis, upper respiratory tract infection, headache, and asthma, with low discontinuation rates due to adverse events (2.1% vs. 3.6% with placebo).2 Serious adverse events were infrequent, with comparable incidences of malignancy and severe infections between tezepelumab and placebo. No treatment-related anaphylaxis was observed.2 Together with its broad efficacy, these findings support tezepelumab as a safe and sustainable long-term treatment option for patients with severe, uncontrolled asthma.4
Conclusion
In summary, the two cases demonstrated tangible clinical benefits with tezepelumab across both T2-high and T2-low asthma phenotypes. By targeting upstream TSLP and modulating multiple inflammatory pathways, tezepelumab offers a novel and comprehensive approach to severe asthma management.2,3 Data from pivotal trials, including NAVIGATOR and pooled NAVIGATOR and PATHWAY analyses, together with real-world evidence, consistently show reductions in exacerbations, improvements in lung function, enhanced symptom control, and potential oral corticosteroid sparing—even among patients previously treated with other biologics.1,2,4 Collectively, these findings position tezepelumab as a transformative therapeutic option that extends disease control beyond biomarker-defined boundaries.3

