CONFERENCE UPDATE: AAAAI 2024
Improving asthma remission with benralizumab in real-world asthma patients in the US: Results from the ZEPHYR-4 study
STUDY DESIGN
The anti-interleukin-5 receptor α (anti-IL-5α) monoclonal antibody (mAb), benralizumab, had been approved for the treatment of severe eosinophilic asthma offering significant improvements in exacerbation rates, maintenance oral corticosteroid (mOCS) use, and other asthma outcomes in phase 3 clinical trials.1 Although benralizumab has achieved asthma remission in clinical trials and non-United States (US) populations, real-world data within the US assessing similar outcomes remained limited.1
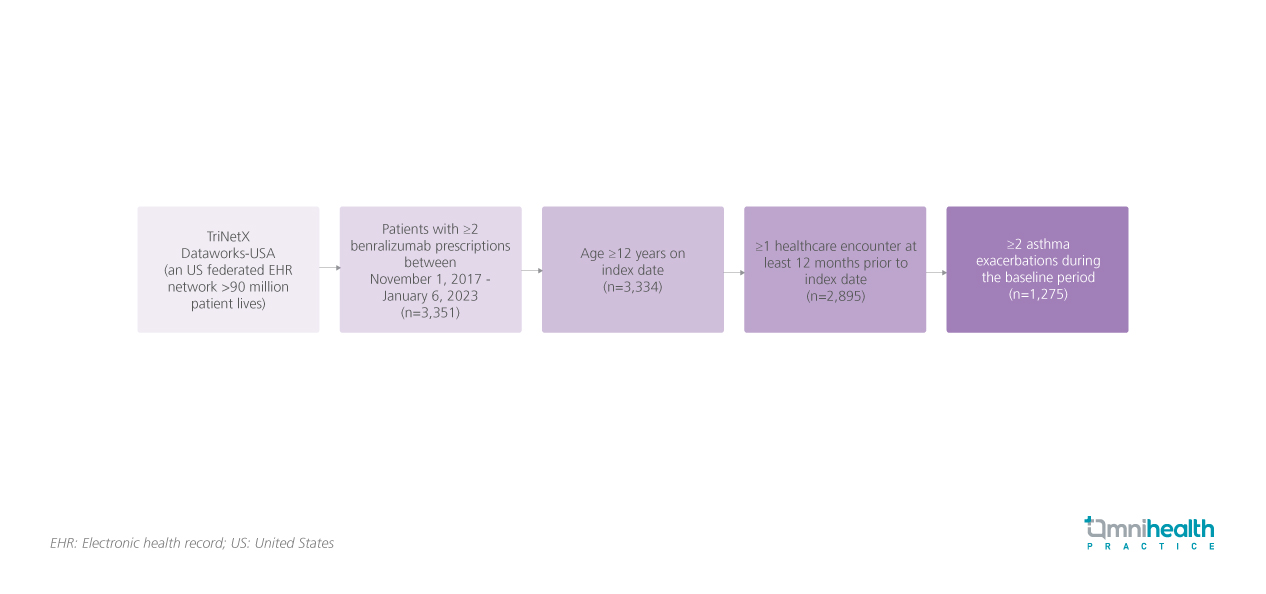
The ZEPHYR-4 study was a retrospective observational study using data from the US federated electronic health record (EHR) describing the clinical outcomes in patients with benralizumab in real-world US practice.1 The study included 2,895 patients aged ≥12 years who had received ≥2 benralizumab prescriptions and had ≥1 healthcare encounter in the previous 12 months.1 A subgroup of patients who have had ≥2 exacerbations (n=1,275) within the 12-month baseline period was also defined and analyzed separately.1
The primary outcome was the proportion of patients achieving the components of remission 12 months after initiating benralizumab.1 The components were established by an expert consensus, and represent a more comprehensive treatment goal for patients with severe asthma.1 Only 3 of the 4 components were assessed in this study as Asthma Control Tests results were unavailable in this dataset.1 The other components included no exacerbations during the 12-month follow-up period, defined as no asthma-related inpatient hospitalization, emergency department visit, or oral corticosteroids (OCS) prescription within 5 days before or after an asthma-related outpatient visit; no mOCS use, defined as ≤4 OCS prescriptions during the 12-month follow-up period; and stabilization of lung function, defined as a ≤10% forced expiratory volume in the first second (FEV1) reduction from baseline.1 Secondary outcomes were blood eosinophil count and lung function changes in the pre- and post-initiation periods.1
FINDINGS
| Primary outcome: |
|
|
|
|
| Secondary outcomes: |
|
|
|
|
|
“This study elucidates real-world benralizumab outcomes by describing key components of asthma remission, an increasingly important asthma endpoint”
Dr. Donna D. Carstens
AstraZeneca,
Wilmington, Delaware, United State

