CONFERENCE UPDATE: ERS 2023
Redefining the lung function parameters in assessing clinical remission of severe asthma with mepolizumab treatment: A post-hoc analysis of the REDES study
STUDY DESIGN
Recent developments in precision medicine and the use of biologics to treat chronic inflammatory diseases, including severe asthma, have led to on-treatment clinical remission (CR) being a plausible treatment goal.1 However, despite the general consensus to evolve the definition of CR in severe asthma from single clinical outcomes to composite measures, the specific outcomes to be included have not been confirmed.1 In this presentation, a post hoc analysis was conducted on the REDES study to assess the impact of including different lung function parameters as the fourth component of CR outcome.1
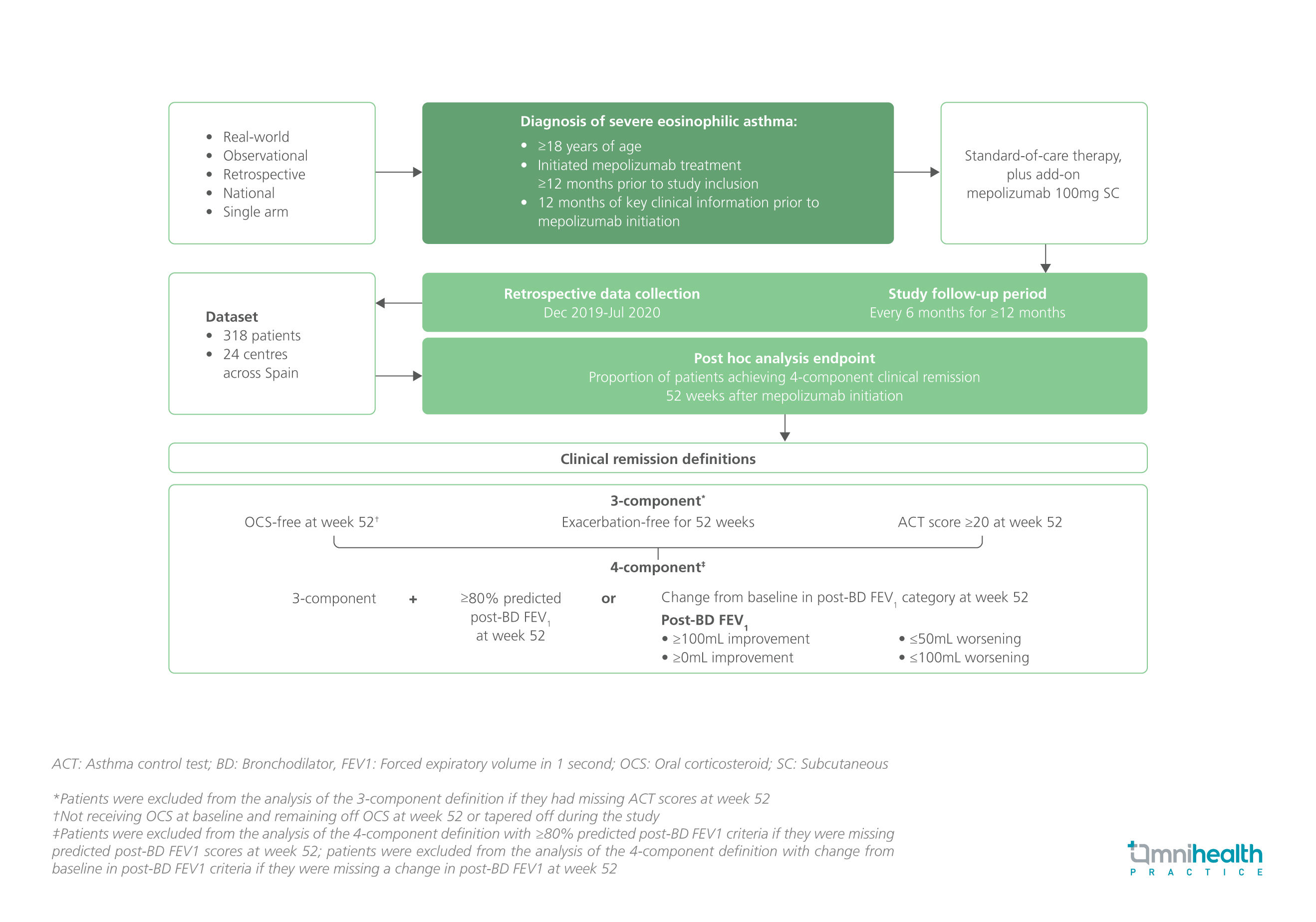
The REDES study was a retrospective, real-world, phase 4, observational study which enrolled 318 adult patients with severe eosinophilic asthma across 24 Spanish hospitals.1 Patients were initiated on mepolizumab and had key clinical information available ≥12 months prior to enrollment.1 These patients received 100mg of mepolizumab subcutaneously (SC) on top of the standard-of-care therapy and were followed up every 6 months for ≥12 months.1
The primary endpoint of the post-hoc analysis was the proportion of patients achieving 4-component clinical remission 52 weeks after mepolizumab initiation which included oral corticosteroid (OCS)-free, exacerbation-free, Asthma Control Test (ACT) score ≥20, and one of the prespecified lung function parameters.1
FINDINGS
| Primary endpoint: |
|
|
|
|
|
"These findings support clinical remission in severe asthma as a realistic real-world goal and should aid the development of a standardized definition of asthma remission on therapy."
Dr. Christian Domingo Ribas
Universitat Autonoma de Barcelona,
Barcelona, Spain

