InhaleAbility Forum
Revealing the role of tiotropium in severity and control of pediatric asthma
Asthma is a common disease that constitutes a serious global health problem and burden on the healthcare systems of societies.1 Despite improvements in asthma management, its prevalence remains high in many countries, especially among younger age groups.1 In a recent symposium organized by the Hong Kong Society of Paediatric Respirology and Allergy, Professor Hon, Kam-Lun Ellis shared his insights on the latest Global Initiative for Asthma (GINA) guidelines on the asthma management in children and adolescents, highlighting the proven benefits of tiotropium on respiratory parameters, symptoms control, and time to exacerbation.
Severity and control of asthma
Asthma is a serious global health problem affecting all age groups, especially children.1 In Hong Kong, asthma accounts for 7.9% and 10% in children aged 6-7 years and 13-14 years, respectively.2 Asthma is categorized based on severity, which will determine the plans for asthma management, ranging from environmental control, fast-acting bronchodilator on demand, inhaled corticosteroids, add-on therapy, to prednisone for very severe disease.1
Professor Hon pointed out that the severity of asthma can be implied to acute exacerbation and control of asthma, referring to as asthma syndromes and persistent asthma. However, critical asthma syndromes could put people at the risk of fatality, which is different from severe asthma in terms of control.3 Fully controlled persistent asthma is a chronic disease where control of symptoms and prevention of exacerbations are targets of chronic disease management.3 Professor Hon mentioned, “One form refers to the control of asthma to divide severity; the other is about the episode itself, how severe it is, and how likely the child may die from asthma.” Hence, patients with good symptom control may experience severe exacerbations, whereas poor symptom control is often associated with an increased risk of exacerbation episodes.1 According to Professor Hon, severe asthma/status asthmaticus (SA) generally means asthma for which the conventional forms of therapy have failed, and it may be life-threatening and severe enough to be admitted to Pediatric Intensive Care Unit (PICU).2 “But severity is not just talking about one acute severe exacerbation, it is about control.”
One of Professor Hon’s studies included 67 admissions of SA patients being admitted to PICU in 2003-2018, showing that SA admissions only contributed to 2.4% of the total PICU admissions, and that non-adherence to prior asthma therapy was very common. 2 Also, results indicated that >80% of patients had a positive result of enterovirus/rhinovirus in the polymerase chain reaction (PCR) test.2
Professor Hon’s another study suggested that better control of asthma was associated with better quality of life (QoL), acceptability of bronchodilators, and negatively with “smokers in family”.4 On the other hand, the worst pediatric allergic disease quality of life questionnaire (PADQLQ) was associated with increased severity of allergic rhinitis, poor control of asthma, increase frequency of Chinese medicine and bronchodilator usage, history of smokers in family, and incense burning at home.4
The current global asthma severity characterization and GINA guidelines
A study in the Netherlands examined the prevalence of severe asthma among Dutch asthmatic patients.5 Results showed that 23.55% of patients received high-intensity treatment with high-dose inhaled corticosteroid (ICS)-long-acting beta-agonist (LABA) or medium-dose ICS-LABA + oral corticosteroids.5 Of these, 17% of cases were classified as difficult-to-treat asthma, among which only 21% were severe refractory asthma, contributing to 3.7% of the Dutch adult asthmatic population.5 Severe asthma was due to a high prevalence of non-adherence to the medication and the application of incorrect inhaler techniques.5
As advised by the GINA guidelines 2022, the treatment goals for asthma are to control symptoms and minimize the risk of asthma-related mortality, exacerbations, and persistent airflow limitations, while limiting the treatment-related side effects.1
The GINA guidelines propose a stepwise management of asthma in children aged 6-11 years by dividing the treatment options into 5 steps.1 Management is based on the severity with escalation in treatments from step 1 to 5.1 Initial treatment includes reliever medication followed by step 1 management with low-dose ICS taken whenever the reliever is taken, or daily low-dose ICS with an as-needed reliever.1 Step 2 recommends the use of daily low-dose ICS as a preferred treatment option, or daily leukotriene receptor antagonist (LTRA) with reliever as needed or low-dose ICS taken whenever the reliever is taken.1 If asthma remains uncontrolled, step 3 suggests the escalation to low-dose ICS-LABA or medium-dose ICS or very low-dose ICS-formoterol maintenance, or low-dose ICS and LTRA, all with reliever medication.1 Step 4 suggests an increase to medium-dose ICS-LABA or low-dose ICS-formoterol maintenance and reliever therapy.1 In this step, tiotropium or LTRA may be added.1 Finally, step 5 involves additional phenotypic assessment, higher doses of medications, and other add-on therapies.1
Studies suggest benefits of tiotropium in severe asthma management
“Tiotropium is a long-acting muscarinic antagonist (LAMA) which has been studied in 18 trials involving more than 6,000 young and adult patients.” Professor Hon highlighted. Pensie-TinA-asthma was a 12-week, phase 3 randomized, double-blind trial that assessed the efficacy and safety of once-daily tiotropium as an add-on to ICS + controller therapy, compared with placebo in adolescents with severe symptomatic asthma.6 Patients aged 12-17 years with ≥3 months history of symptomatic asthma and on maintenance treatment with high-dose ICS + ≥1 controller or medium-dose ICS + ≥2 controllers were included.6 A total of 392 patients were randomized 1:1:1 to receive tiotropium 2.5μg, 5μg, or placebo.6 The primary efficacy endpoint was the peak forced expiratory volume in the first second (FEV1) within 3 hours post-dosing (FEV1(0-3h)) measured as a response, which was defined as a change from baseline.6 The key secondary efficacy endpoint was the trough FEV1 response.6
Despite an improvement of 90mL in peak FEV1(0-3h) response compared with placebo, patients receiving tiotropium 5μg did not have a statistically significant improvement from baseline (95% CI: -19 to 198; p=0.104), while patients receiving tiotropium 2.5μg showed a statistically significant improvement of 111mL in peak FEV1(0-3h) response compared with placebo (95% CI: 2-220; p=0.046).6 Trough FEV1 response was also numerically improved by 54mL and 115mL in the tiotropium 5μg and 2.5μg, respectively, compared with placebo, but was not statistically significant.6
Similarly, VivaTinA-asthma was a 12-week, double-blind, phase 3 trial conducted in children with severe symptomatic asthma to examine the efficacy and safety of once-daily tiotropium as an add-on to ICS + controller therapy compared with placebo.7 A total of 401 patients aged 6-11 years on at least high-dose ICS + ≥1 controller or medium-dose ICS + ≥2 controllers were randomized 1:1:1 to receive tiotropium 2.5μg, 5μg, or placebo.7 The primary endpoint was the peak FEV1(0-3h), and the key secondary endpoint was trough FEV1, both at 12 weeks.7
Results showed that tiotropium at a dose of 5μg demonstrated a statistically significant increase compared with placebo in peak FEV1(0-3h) response by 139mL (95% CI: 75-203; p<0.001) and in trough FEV1 by 87mL (95% CI: 19-154, p<0.05).7 However, the increase in peak FEV1(0-3h) by 35mL (95% CI: -28 to 99, p=0.27) and trough FEV1 by 18mL (95% CI: -48-85, p=0.59) in the tiotropium 2.5μg, when compared with placebo, was not statistically significant.7
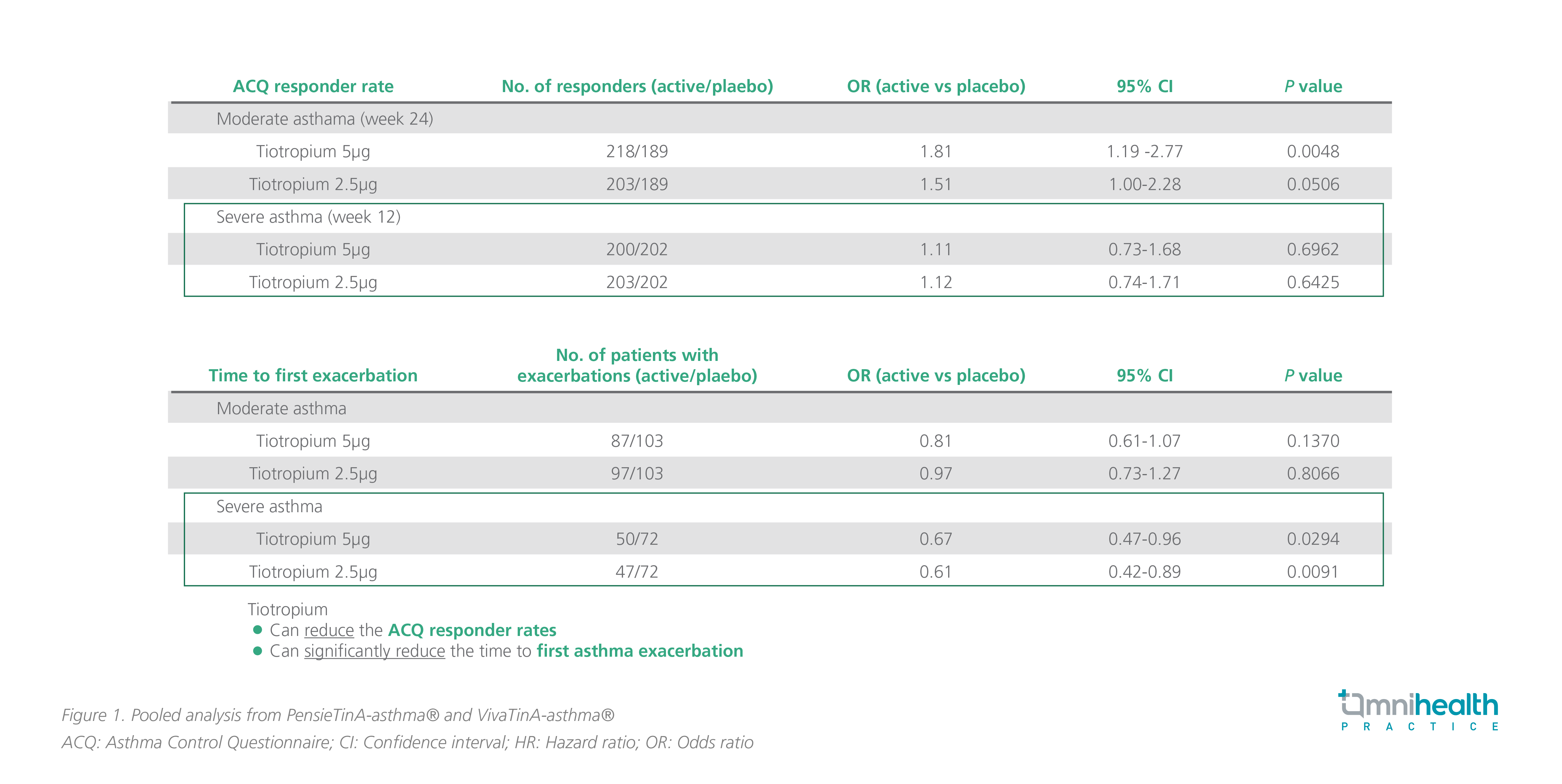
Data from a pooled analysis of phase 3 trials of Pensie-TinA-asthma, VivaTinA-asthma, Cano TinA-asthma, and RubaTinA-asthma, including patients with symptomatic moderate or severe asthma, were used to explore whether the responses of tiotropium add-on were affected by patients’ type 2 status, measured by the serum immunoglobulin E (IgE) levels and blood eosinophil counts.8 The primary endpoint of each study was the peak FEV1(0-3h), measured as the response at week 24 for those with moderate symptomatic asthma, and at week 12 for those with severe symptomatic asthma.8 The key secondary endpoints included trough FEV1 response at week 24 for symptomatic moderate asthma or week 12 for severe symptomatic asthma, forced expiratory flow at 25%-75% of the pulmonary volume (FEF25%-75%) response, asthma control questionnaire (ACQ), and investigator-administered ACQ (in children aged 6-11 years) responder rate, and time to first asthma exacerbation.8
Both doses of tiotropium demonstrated significantly greater improvements in peak FEV1(0-3h) response vs. placebo in moderate and severe asthma.8 Patients with severe asthma had an increase of 117mL and 74mL in peak FEV1(0-3h) in the tiotropium 5μg and 2.5μg groups, respectively (95% CI: 51-183, p=0.0005 and 95% CI: 8-140, p=0.0273, respectively).8 Moreover, trough FEV1 and FEF25%-75% at week 12 for patients with severe symptomatic asthma were statistically and significantly improved compared with placebo, except for trough FEV1 at a tiotropium dose of 2.5μg.8 In severe asthma, The ACQ responder rates were higher among patients receiving tiotropium 5μg or 2.5μg than patients receiving placebo (OR=1.11; 95% CI: 0.73-1.68; p=0.6962 and OR=1.12; 95% CI: 0.74-1.71; p=0.6425, respectively) (figure 1).8 Both tiotropium doses led to a statistically significant reduction in the time to first asthma exacerbation vs. placebo in severe asthma (HR=0.67; 95% CI: 0.47-0.96; p=0.0294 for tiotropium 5μg; HR=0.61; 95% CI: 0.42-0.89; p=0.0091 for tiotropium 2.5μg).8
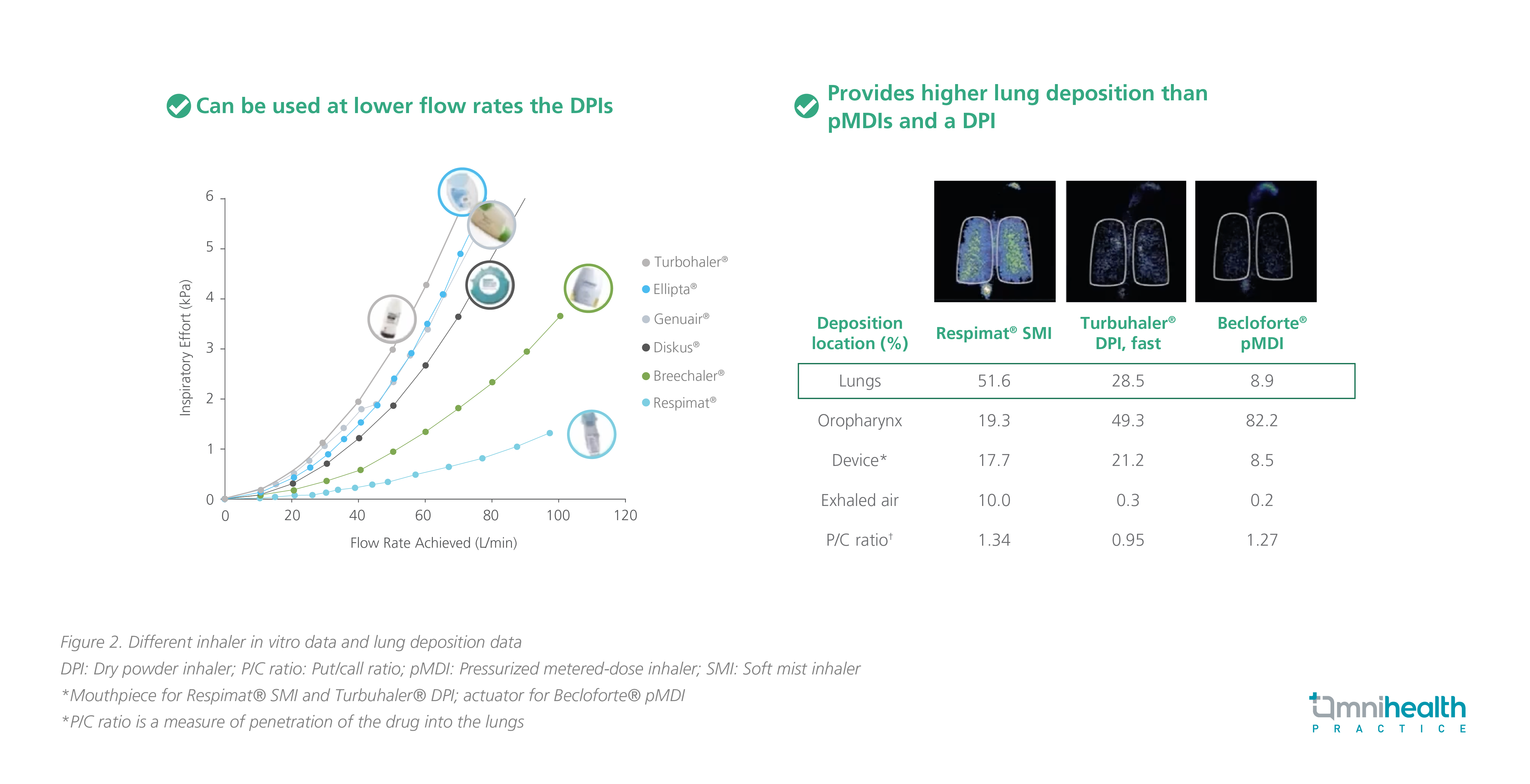
Respimat® as an ideal inhaler device
Tiotropium comes in the form of Respimat® inhaler, and this type of inhaler can deliver a consistent dose at high and low flow rates requiring lower inspiratory efforts than dry powder inhalers (DPI), such as Turbohaler®, Ellipta®, Genuair®, Diskus®, and Breezhaler® (figure 2).9 Among the different inhalers available, Respimat® soft mist inhaler provides a higher lung deposition (51.6%) compared with Turbohaler® DPI (28.5%), and Becloforte™ metered-dose inhaler (8.9%).10
Respimat® is suitable for children aged <5 years and can be used by themselves when provided with simple training.11 It can be also used with AeroChamber® for children who have difficulties in using inhalers alone.11
Conclusion
To conclude, it is advised to assess patients’ asthma severity, asthma control, and QoL, and get to know the current GINA guidelines and inhaler medications to optimize treatment for asthma patients, especially children and adolescents. If a patient’s asthma remains uncontrolled and progresses to moderate-to-severe asthma, tiotropium, a LAMA, is considered a treatment option due to its proven benefits in severe asthma, according to steps 4-5 of the GINA guidelines. Besides, Respimat®, an optimal inhaler device that delivers consistent and desired therapeutic effects, is recommended for improving treatment outcomes.
This is an independent editorial article, published and distributed through unrestricted educational support from the pharmaceutical community, for the purpose of continuing medical education only. The views expressed in this publication reflect the experience and/or opinion of the author(s) and are not necessarily those of editors, publisher and sponsor(s). Because of rapid advances in medicine, independent verification of clinical diagnoses, medical suitability and dosage should be made before treatment prescription. The appearance of advertisement, if any, has no influence on editorial content or presentation and does not imply the endorsement of products by the publication, or its authors and editors.
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2022. https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf. Accessed August 21, 2022
- Cheng WT et al. Outcome of status asthmaticus at a pediatric intensive care unit in Hong Kong. Clin Respir J. 2020;14(5):462-470.
- Kenyon N et al. Definition of critical asthma syndromes. Clin Rev Allergy Immunol. 2015;48(1):1-6.
- Hon KL et al. Determinants for asthma control, quality of life and use of complementary and alternative medicine in asthmatic pediatric patients in four cities. World J Pediatr. 2018;14(5):482-491.
- Hekking PW et al. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896-902.
- Hamelmann E et al. A randomized controlled trial of tiotropium in adolescents with severe symptomatic asthma. Eur Respir J. 2017;49(1):1601100.
- Szefler SJ et al. A phase III randomized controlled trial of tiotropium add-on therapy in children with severe symptomatic asthma. J Allergy Clin Immunol. 2017;140(5):1277-1287.
- Szefler SJ et al. Tiotropium Is Efficacious in 6- to 17-Year-Olds with Asthma, Independent of T2 Phenotype. J. Allergy Clin. Immunol. Pract. 2019;7(7):2286-2295.e4.
- Ciciliani A et al. In vitro dose comparison of Respimat® inhaler with DPI for COPD maintenance therapy. Int J Chron Obstruct Pulmon Dis. 2017;12:1565-1577.
- Pitcairn G et al. Deposition of corticosteroid aerosol in the human lung by Respimat Soft Mist inhaler compared to deposition by MDI or by Turbuhaler DPI. J Aerosol Med. 2005;18(3):264-272.
- Kamin W et al. A Handling Study to Assess Use of the Respimat® Soft Mist™ Inhaler in Children Under 5 Years Old. J. Aerosol Med. Pulm. Drug Deliv. 2015;28(5):372-381.

