Chronic kidney disease (CKD) is estimated to affect over 850 million individuals worldwide, with global prevalence estimated at 9.1%.1 CKD often remains under-recognized, underdiagnosed, and undertreated.1 At a recent webinar, Professor Adeera Levin from the University of British Columbia, Canada, emphasized the urgent need for earlier identification and intervention in CKD. She highlighted the pivotal role of sodium-glucose co-transporter 2 (SGLT2) inhibitors, particularly empagliflozin, which have shown significant benefits in reducing the risk of CKD progression or death from cardiovascular (CV) causes across a broad spectrum of CKD populations regardless of diabetes status.1,2 The discussion underscored the importance of risk stratification and early system-wide intervention to address the interconnected risks of kidney and heart health, ultimately improving clinical outcomes.1
The growing burden of CKD: A global health crisis
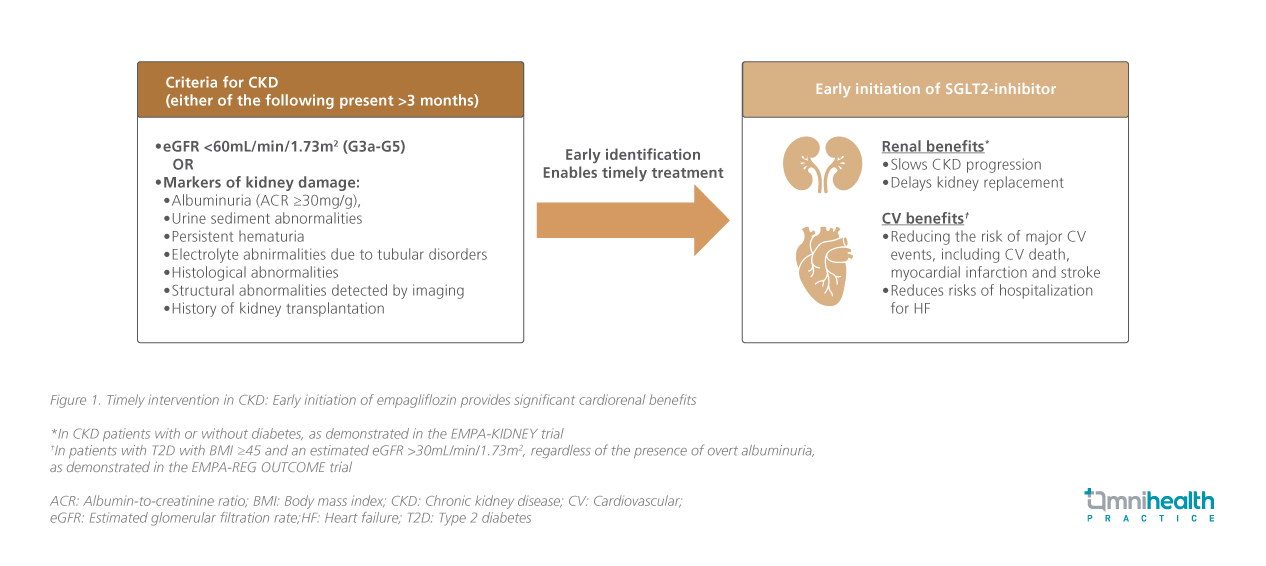
CKD is defined by the presence of either kidney damage markers or decreased glomerular filtration rate (GFR <60mL/min/1.73 m²; G3a-G5) persisting for at least three months (figure 1).1 CKD imposes a significant global health burden and is a leading cause of death and disability, with its effect on CV diseases accounting for 2.6 million deaths globally, largely driven by aging populations and the increasing prevalence of risk factors such as diabetes and hypertension.1 As seen in a large cohort study of Chinese adults with type 2 diabetes (T2D) in Hong Kong, the incidence of end-stage renal disease (ESRD) increases with the duration of diabetes.3 Since 1997, diabetes mellitus (DM) has been the leading cause of kidney failure requiring kidney replacement therapy (KRT) in Hong Kong, accounting for 51.5% of the cases in 2022.4
CKD as a CV risk multiplier: The role of albuminuria and reduced eGFR
Even mild reductions in estimated glomerular filtration rate (eGFR) and elevations in albuminuria are independently associated with increased risks of cardiorenal events.1,5 Patients with both urine albumin-to-creatinine ratio (UACR) ≥300mg/g and eGFR <60mL/min/1.73m² face up to a 3.2-fold higher risk of CV events and a 22.2-fold higher risk of renal events compared to those with neither of these risk factors.5
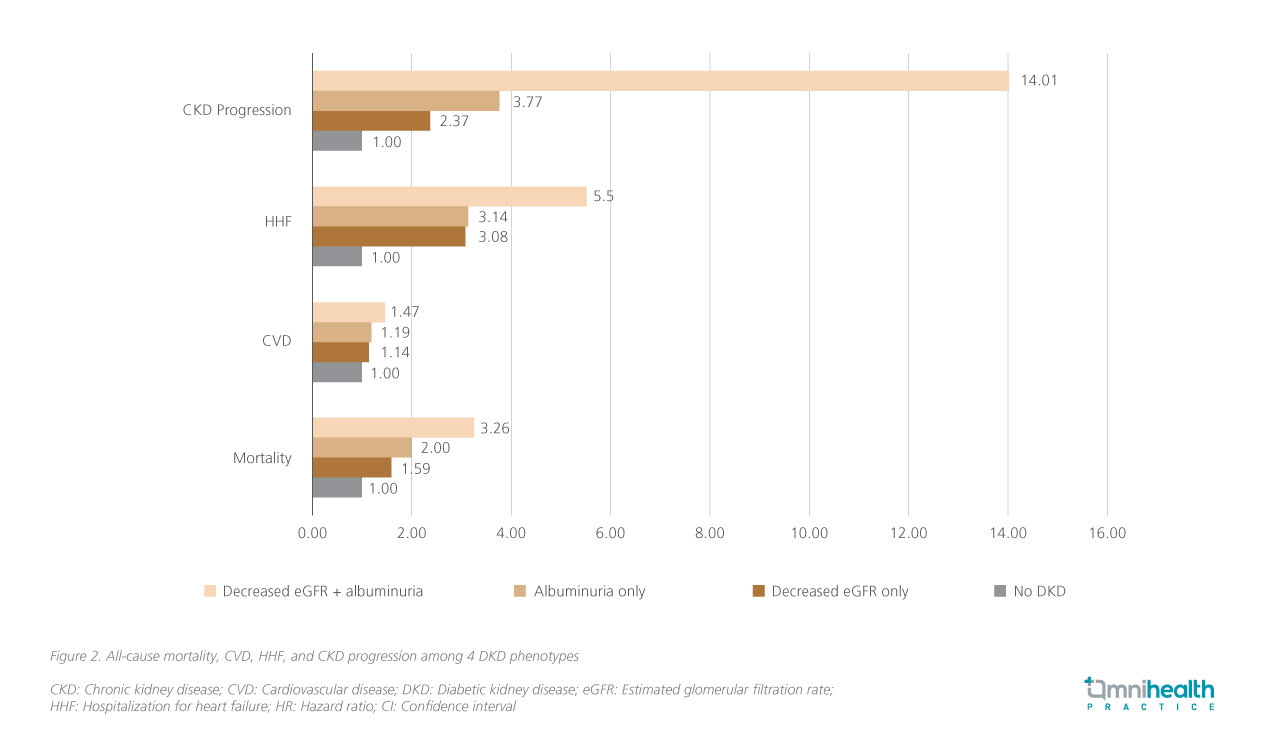
Notably, recent data demonstrated that non-albuminuric diabetic kidney disease (DKD), which is more commonly observed in older adults, women, and individuals with a history of CV disease, also heightens the risk of CV disease and hospitalization for heart failure (HHF), and CKD progression (figure 2).6 The Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicentre study further reported that reduced eGFR without albuminuria was independently associated with a significant CV burden.7 Collectively, these data found that normoalbuminuric DKD is associated with adverse CV and renal outcomes.6,7 As Prof. Levin remarked, “CKD acts as a CV risk multiplier, even with low levels of albuminuria.”
CKD risk assessment: Beyond eGFR alone
CKD frequently coexists with other chronic conditions, affecting 20%-67% of patients with HF and approximately 37% of adults with diabetes, where its presence increases the mortality risk by 25%-28%.8-10 These overlapping burdens drive progressive organ damage, excess mortality, and increased healthcare utilization.11 Despite the high burden, 53% of patients with T2D had no urine ACR assessment and 15% lacked eGFR evaluation, representing missed opportunities for timely intervention.12
Risk stratification tools such as the KDIGO heat map and Kidney Failure Risk Equation (KFRE) enable more accurate identification of individuals at increased risk of CKD progression, CV events, and mortality, by incorporating factors beyond eGFR alone, including ACR, age, and sex.1 The CKD Prognosis Consortium (CKD-PC) models and PREVENT equations further refine risk prediction, supporting individualized management through more precise estimation of kidney and CV outcomes.1 These tools enhance clinical decision-making, patient counseling and treatment planning.1 As Prof. Levin emphasized, “These tools allow us to move beyond arbitrary eGFR cutoffs and identify who truly needs early referral, multidisciplinary care, or preparation for dialysis.”
Empagliflozin has demonstrated efficacy across the CKD spectrum
Optimizing risk factors, including glycemic control, blood pressure, lipid management, and dietary interventions, alongside the use of evidence-based therapies such as renin-angiotensin-aldosterone system (RAAS) inhibitors and SGLT2 inhibitors, is fundamental to mitigating CKD progression and its CV complications.1,2 As one of the SGLT2 inhibitors available, empagliflozin is supported by evidence across a broad spectrum of CKD populations, including those with early-stage, low albuminuria, and non-diabetic CKD (figure 3).2
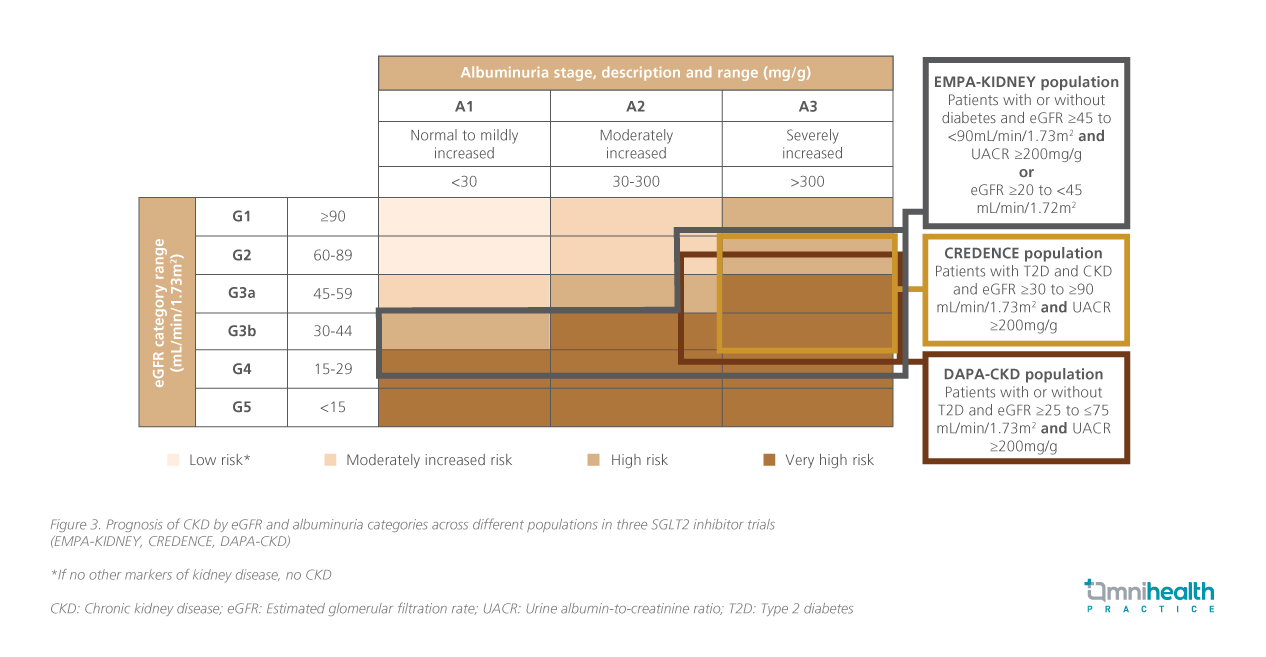
The EMPA-KIDNEY trial, which enrolled more than 6,600 participants with varying degrees of kidney function and albuminuria, demonstrated a 28% reduction in CKD progression or CV death with empagliflozin compared to those in the placebo group (HR=0.72; 95% CI: 0.64-0.82, p<0.001), regardless of baseline diabetes status.2 Notably, empagliflozin consistently slowed eGFR decline by ~50% vs. placebo, reinforcing its renoprotective effect.2 These benefits extended across key subgroups, including older individuals, those with frailty, multimorbidity, and polypharmacy.2,13 Prof. Levin highlighted, “These benefits extend beyond glycemic control, slowing kidney function decline across a broad range of patients with CKD — the kind of people we see every day. What matters to patients is how fast their kidneys are changing now, not just dialysis far in the future.”
Comprehensive cardiorenal benefits of SGLT2 inhibitors
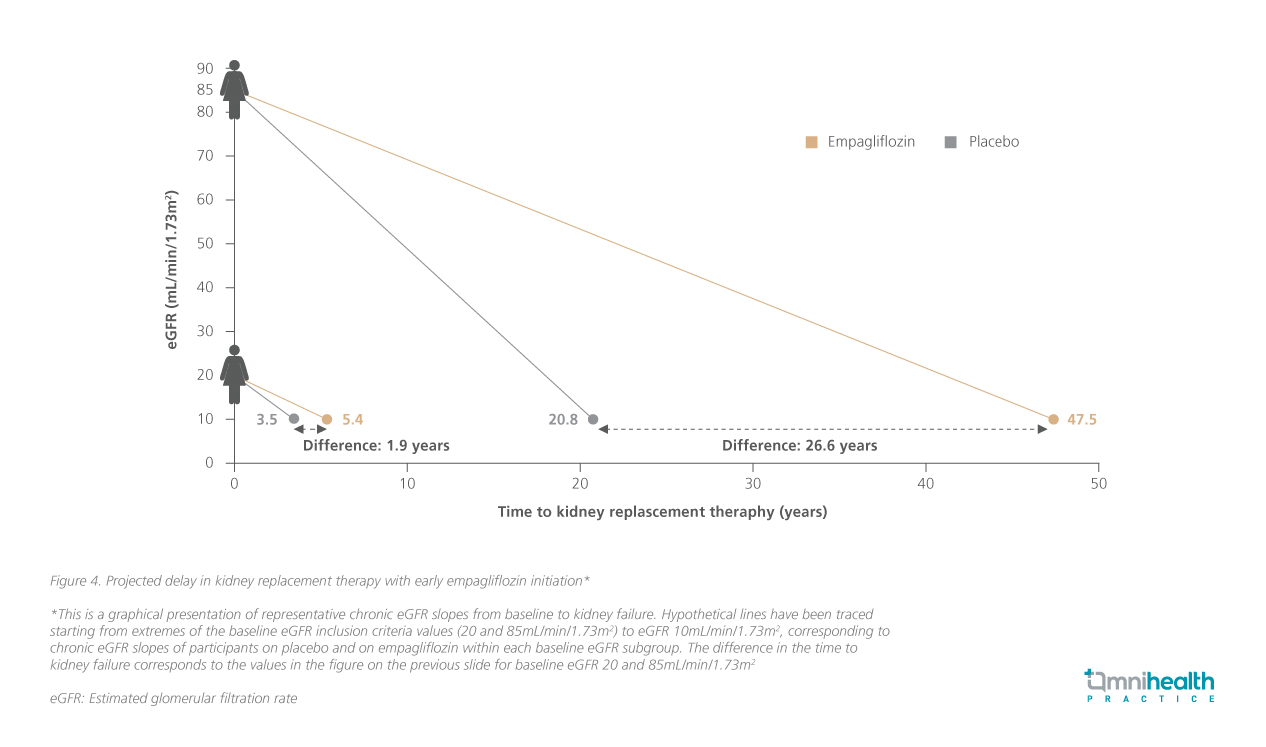
Early initiation of empagliflozin is also estimated to delay the need for KRT by more than 26 years (figure 4).14 Prof. Levin emphasized, “This reflects a hypothetical shift in the trajectory of kidney function decline, potentially giving people another 2 to 26 years before needing kidney replacement therapy.”
As seen in the EMPA-REG OUTCOME trial, empagliflozin has demonstrated significant CV and renal benefits in patients with T2D at high CV risk.15 Compared with placebo, empagliflozin significantly reduced the risk of the primary composite outcome of CV death, nonfatal myocardial infarction, or nonfatal stroke (HR=0.86; 95% CI: 0.74-0.99; p=0.04), death from CV causes (HR=0.62; 95% CI: 0.49-0.77; p<0.001), hospitalization for HF (HR=0.65; 95% CI: 0.50-0.85; p=0.002), and incident or worsening nephropathy (HR=0.61; 95% CI: 0.53-0.70; p<0.001).15,16 In a sub-analysis of the trial, it was shown that empagliflozin’s CV and renal benefits were consistent regardless of the presence of overt albuminuria.17
Consistent with these findings, the KDIGO 2024 guidelines recommend SGLT2 inhibitors as first-line therapy in CKD, irrespective of diabetes status, for their CV and kidney benefits.1 The guidelines recommend that CKD patients with eGFR ≥20mL/min/1.73m² and UACR ≥200mg/g, patients with eGFR 20-45mL/min/1.73m² with UACR <200mg/g, and patients with HF irrespective of albuminuria level, should be treated with SGLT2 inhibitors as first-line therapy.1 Combination therapy with glucagon-like peptide-1 (GLP-1) receptor agonists may further reduce CV and HF future events, particularly in CKD patients with high CV risk.18 Other agents, such as non-steroidal mineralocorticoid receptor antagonists (ns-MRAs), are also recommended for selected patients with CKD and T2D who have persistent albuminuria despite optimized renin-angiotensin system inhibitor (RASi) use, to further reduce the risk of CKD and CV events.1,19
Conclusion
The KDIGO guidelines advocate early initiation of SGLT2 inhibitors, such as empagliflozin, in patients with CKD irrespective of diabetes status, to reduce the risk of CKD progression and CV events.1 A comprehensive approach is recommended to address the interconnected risks within the CKM framework.18 Treatment decisions should consider clinical indications, benefit-risk profiles, potential nephrotoxicity, as well as accessibility, affordability, and patient preferences.1 Prof. Levin highlighted: “Understanding CKD, DKD, and individualizing patient risks is essential. Early interventions protect hearts and kidneys, and maintaining optimal therapy requires regular review of clinical and laboratory parameters. Ultimately, we are saving kidneys, hearts, and lives.” Integrating guideline-based therapies with patient-centered care is key to improving long-term outcomes in CKD management.1
This is an independent editorial article, published and distributed through unrestricted educational support from the pharmaceutical community, for the purpose of continuing medical education only. The views expressed in this publication reflect the experience and/or opinion of the author(s) and are not necessarily those of editors, publisher, and sponsor(s). Because of rapid advances in medicine, independent verification of clinical diagnoses, medical suitability and dosage should be made before treatment prescription. The appearance of advertisement, if any, has no influence on editorial content or presentation and does not imply the endorsement of products by the publication, or its authors and editors.
- Kidney Disease: Improving Global Outcomes (KDIGO) 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024; 105(6S): S1-S150.
- The EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023; 388: 117-27.
- Luk AOY, et al. Declining trends of cardiovascular-renal complications and mortality in type 2 diabetes: The Hong Kong Diabetes Database. Diabetes Care. 2017; 40:9 28-935.
- Chan JYH et al. The Hong Kong Renal Registry: a recent update. Hong Kong Med J. 2024; 30: 332-336.
- Ninomiya T et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009; 20: 1813-1821.
- Jin Q et al. Nonalbuminuric diabetic kidney disease and risk of all-cause mortality and cardiovascular and kidney outcomes in type 2 diabetes: findings from the Hong Kong Diabetes Biobank. Am J Kidney Dis. 2022; 80: 196-206.
- Pugliese G et al. Chronic kidney disease in type 2 diabetes: Lessons from the Renal Insufficiency and Cardiovascular Events (RIACE) Italian Multicentre Study. Nutr Metab Cardiovasc Dis. 2014; 24: 815-822.
- Sarraf M et al. Cardiorenal syndrome in acute decompensated heart failure. Clin J Am Soc Nephrol. 2009; 4:2 013-2026.
- Ather S et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012; 59: 998-1005.
- Murphy D et al. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med. 2016; 165: 473-481.
- Siemens Healthineers. Chronic kidney disease: a global crisis. 2018. Available at: https://www.siemens-healthineers.com/en-my/news/chronic-kidney-disease.html. Accessed July 2025.
- Szczech LA et al. Primary care detection of chronic kidney disease in adults with type 2 diabetes: the ADD-CKD study (Awareness, Detection and Drug Therapy in Type 2 Diabetes and Chronic Kidney Disease). PLoS One. 2014; 9: e110535.
- Mayne KJ et al. Frailty, multimorbidity, and polypharmacy: Exploratory analyses of the effects of empagliflozin from the EMPA-KIDNEY trial. Clin J Am Soc Nephrol. 2024; 19: 1119-1129.
- Fernández-Fernández B et al. EMPA-KIDNEY: Expanding the range of kidney protection by SGLT2 inhibitors. Clin Kidney J. 2023; 16(8): 1187-1198.
- Zinman B, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373(22): 2117-2128.
- Wanner C, et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375(4):323-334.
- Wanner C, et al. Consistent effects of empagliflozin on cardiovascular and kidney outcomes irrespective of diabetic kidney disease categories: Insights from the EMPA-REG OUTCOME trial. Diabetes Obes Metab. 2020;22(12):2335-2347.
- Ndumele CE et al. A Synopsis of the Evidence for the Science and Clinical Management of Cardiovascular-Kidney-Metabolic (CKM) Syndrome: A Scientific Statement From the American Heart Association. Circulation. 2023; 148: 1636-1664.
- Agarwal R et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022; 43: 474-484.


