CONFERENCE UPDATE: CROI 2025
B/F/TAF maintains HIV-1 and c suppression after switch from DTG + F/TDF: 48-week results from ALLIANCE OLE
STUDY DESIGN
Approximately 3.1 million people worldwide live with concomitant human immunodeficiency virus-1 (HIV-1) and hepatitis B virus (HBV) infection.1 Current guidelines recommend tenofovir alafenamide (TAF) or tenofovir disoproxil fumarate (TDF)-based antiretroviral therapies for most adults and adolescents with HIV-1 and HBV co-infection.1 The ALLIANCE study previously demonstrated that bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) was noninferior to dolutegravir (DTG) + F/TDF for HIV-1 ribonucleic acid (RNA) suppression and superior for HBV DNA suppression at week 48 in treatment-naïve adults.1 These suppression rates were sustained through week 96.1 Following this, participants in the DTG + F/TDF arm had the option to switch to B/F/TAF for an additional 48 weeks of open-label extension (OLE).1 The captioned analysis evaluated the efficacy and safety of B/F/TAF in treatment-experienced adults who transitioned from DTG + F/TDF during the OLE phase.1
ALLIANCE was a phase 3, randomized, double-blind, active-controlled clinical trial comparing B/F/TAF to DTG + F/TDF in adults with HIV-1 and HBV.1 The captioned analysis focuses on 89 participants who switched to B/F/TAF after completing at least 96 weeks of the randomized phase, reporting outcomes from OLE baseline to week 48.1 At OLE baseline, 98% of participants were male at birth, and 94% were Asian.1 The median age was 34 years (interquartile range [IQR]: 28-39 years).1 Elevated alanine aminotransferase (ALT) levels were observed in 30% of participants, and 58% were HBeAg-positive.1 At the time of switching, 96.6% had HIV-1 RNA suppressed to less than 50 copies (c)/mL, while 83.1% had HBV DNA levels below 29IU/mL.1 By week 48 of the OLE phase, 98.9% of participants (n=88) who switched to B/F/TAF successfully completed treatment.1 The key outcomes assessed included HIV-1 RNA and HBV DNA suppression, ALT normalization based on American Association for the Study of Liver Diseases (AASLD) 2018 criteria, HBeAg and HBsAg loss or seroconversion, and safety assessments.1
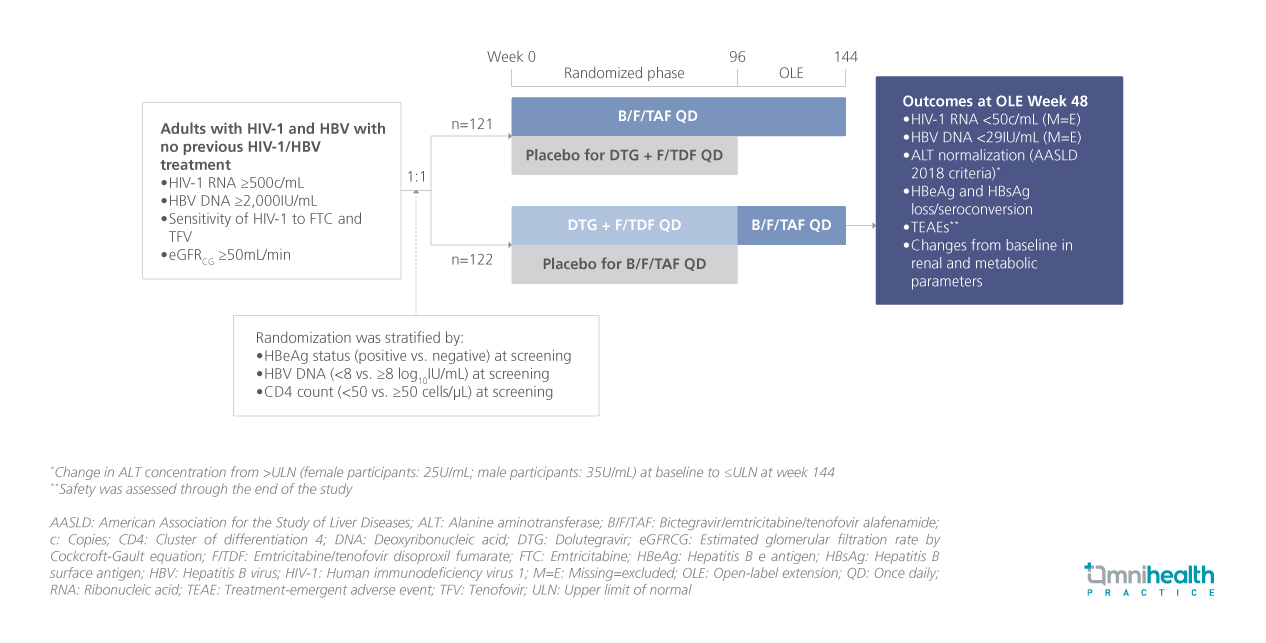
FINDINGS
|
Efficacy outcomes: |
|
|
|
|
| Safety: |
|
|
|
|
|
|
"Through 48 weeks of the OLE phase, B/F/TAF maintained high rates of HIV-1 and HBV virologic suppression following switch from DTG + F/TDF"
Dr. Anchalee Avihingsanon
HIV-NAT,
Thai Red Cross AIDS and Infectious Disease Research Centre,
Bangkok, Thailand

