CONFERENCE UPDATE: AAN 2024
Interim analysis of a long-term safety and efficacy extension study of atogepant in episodic and chronic migraine prevention
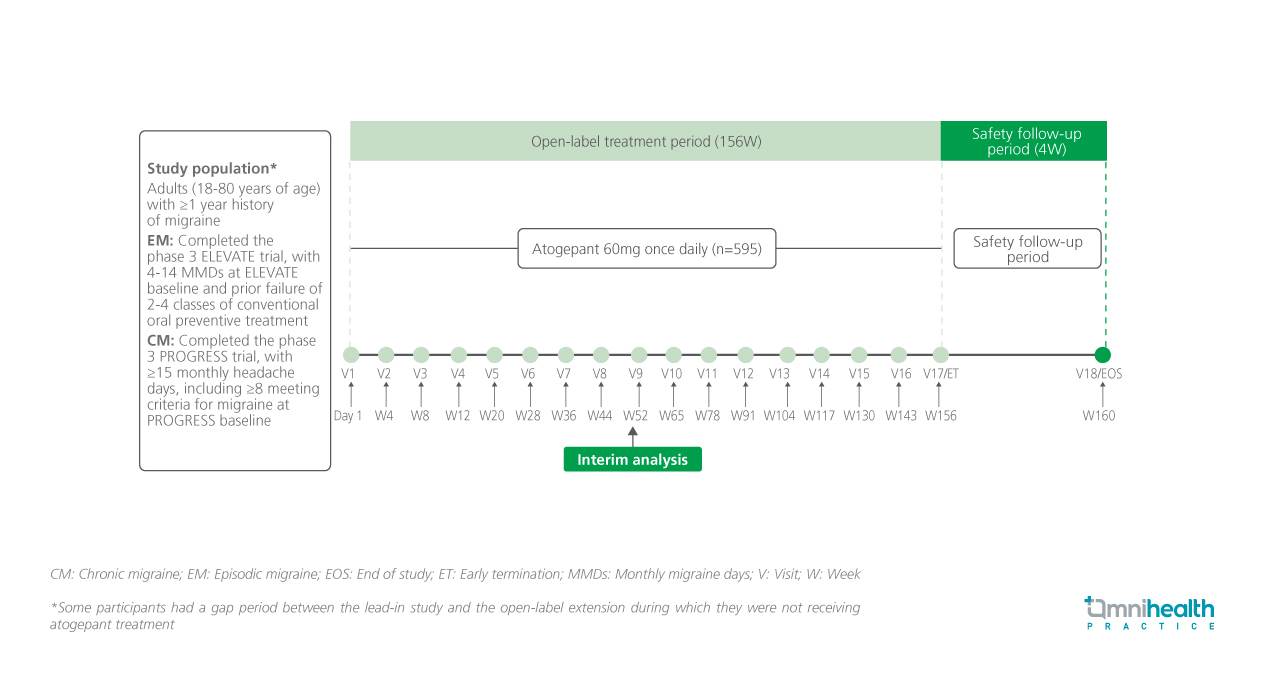
STUDY DESIGN
Atogepant is an oral anti-migraine drug that blocks the calcitonin gene-related peptide (CGRP) receptor.1 It was previously evaluated in the phase 3, randomized, double-blind, placebo-controlled trials ELEVATE and PROGRESS for the prevention of episodic migraine (EM) or chronic migraine (CM), respectively.1
In the captioned, ongoing, multicenter, open-label long-term safety extension study (3101-312-002), atogepant 60mg once daily (QD) was given over a 156-week period for the preventive treatment of EM or CM.1 All 595 adult participants (18-80 years of age) with ≥1 year history of migraine had completed the trials (ELEVATE, n=270; PROGRESS, n=325).1 This interim analysis used efficacy data collected at week 52, including those who withdrew from the study, to evaluate the long-term safety, tolerability and efficacy in EM and CM prevention.1
The efficacy outcomes included changes from the lead-in study baseline in monthly migraine days (MMDs), monthly headache days, monthly acute medication use days and the proportion of participants with ≥50% reduction in MMDs.1 These data were presented in eDiary and were collected at weeks 13-16, 29-32 and 45-48.1 The baseline number of MMDs in the lead-in study was 19.2 (PROGRESS), 9.1 (ELEVATE) and 14.5 (total).1 This was evaluated in the modified intent-to-treat (mITT) population which included 524 participants who received at least one dose of atogepant and ≥1 evaluable post-baseline 4-week period of eDiary data.1 The safety profile of atogepant was also assessed.1 All participants who were administered at least one dose of the study intervention drug were included in the safety population (n=595).1
FINDINGS
|
Efficacy outcomes: |
|
|
|
|
|
Safety: |
|
|
|
|
|
|
|
“This is very encouraging for clinicians, suggesting that atogepant is not only safe for at least one year but it can also be considered for the long-term prevention of migraine”
Dr. Sait Ashina
Comprehensive Headache Center,
Beth Israel Deaconess Medical Center,
Boston, United States

