CONFERENCE UPDATES: AAN 2024
Observational FINESSE study of real-world effectiveness and tolerability of fremanezumab in episodic and chronic migraine
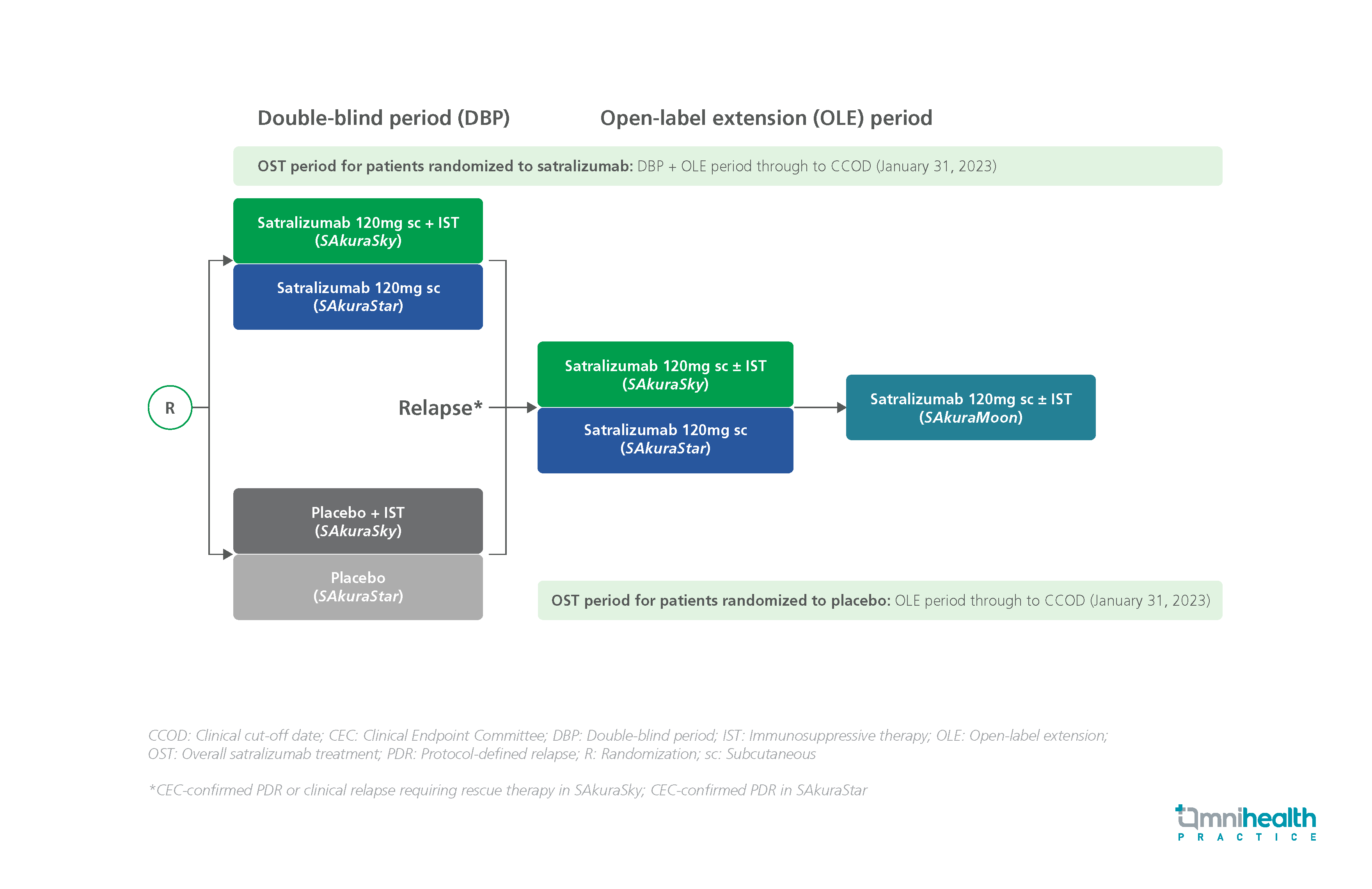
STUDY DESIGN
Fremanezumab is an anti-migraine agent that has been shown to be effective and well-tolerated in patients with episodic migraine (EM) and chronic migraine (CM).1 Real-world data are required to support these clinical findings in real-world populations.1
FINESSE is an ongoing, non-interventional, multi-center, two-country (Germany and Austria), prospective, 24-month, observational study conducted to evaluate the real-world effectiveness and tolerability of fremanezumab in EM and CM prevention.1 The study included a total of 926 patients, with a slightly higher proportion of EM patients (56.5%) compared with those who had CM (43.5%).1 The patients had a mean age of 45.6, and the majority (89.3%) were female.1 This third interim analysis of the FINESSE study evaluated outcomes from adult patients with either EM or CM who were receiving fremanezumab treatment as part of their routine clinical management for up to 1 year.1 The analysis of effectiveness was based on data obtained from patient diaries and patient-reported outcome measures.1
The primary endpoint of the FINESSE study was the proportion of patients who experienced ≥50% reduction in the average number of monthly migraine days (MMDs) over the first 6 months following the initiation of fremanezumab treatment.1 Secondary endpoints included changes in the average number of MMDs, disability scores assessed by Migraine Disability Assessment (MIDAS), 6-Item Headache Impact Test (HIT-6) and other acute migraine medication utilization alongside fremanezumab.1 Safety data were collected based on the documentation of adverse events (AEs) reported during the routine clinical management of patients.1
FINDINGS
|
Primary endpoint: |
||||
|
||||
|
Secondary endpoints: |
||||
|
||||
|
Safety: |
||||
|
||||
"Sustained reductions in MMD, disability and acute medication use were observed at 6 and 12 months post-fremanezumab initiation"
Dr. Mario Ortega
Teva Pharmaceuticals

