FEATURES
Examining real-world effectiveness of fremanezumab in migraine prevention: Insights from the 3rd interim analysis of PEARL
Migraine is a neurologic disorder characterized by recurrent attacks of pulsating headache that may be accompanied by an array of sensory symptoms or even cognitive dysfunction.1,2 Traditional treatments for migraine prevention are rarely disease-specific with unsatisfactory effectiveness and tolerability profiles, leading to poor treatment adherence and persistence.3 Fremanezumab has previously demonstrated its efficacy and safety in patients suffering from migraines in the clinical trial setting.3 Since its approval for migraine prevention among patients with ≥4 mean migraine days (MMD), data accumulated post-approval have shown similar results sustained over longer periods among real-life populations.4
The unrelenting burden of migraine
Migraine is a highly disabling disease with a global prevalence of up to 18%.1 Over the course of just one year, headache disorders such as migraine have accounted for >46 million years lived with disability worldwide.3 It is classified into episodic migraine (EM), defined as headaches occurring for <15 days per month, or chronic migraine (CM), where headaches occur for ≥15 days per month over ≥3 months.2 EM patients are at risk of developing CM with approximately 30% of patients reporting ≥1 episode per week.2
The disease burden of migraines is high, affecting many aspects of patients’ lives, incurring a high cost at a societal level as a result.3 From the patients’ perspective, there is a substantial reduction in quality of life (QoL), brought on not only by the migraine attacks but also the anticipatory anxiety and depression between episodes.3 Despite consensus on the need for prevention, preventive treatment is often underused or not adhered to that in reality due to ineffectiveness or adverse effects (AEs), especially among patients with EM.1-3
Offer reliable reprieve with fremanezumab
Calcitonin gene-related peptide (CGRP) humanized monoclonal antibody (mAb) such as fremanezumab potently and selectively binds to the CGRP ligand, representing a new avenue of treatment for both EM and CM patients.3 It is administered subcutaneously with a flexible dosing regimen of 225mg every month or 675mg every three months.1,2 Clinical data of fremanezumab use over 12 weeks of treatment have demonstrated its safety and efficacy among both EM and CM patients.1,2 Further investigations into populations with acute medication overuse and those with inadequate response to up to four classes of migraine preventive medications further substantiates its utility.3 Patients had also reported high levels of treatment satisfaction with respect to various QoL measures.3
To further assess its use, the Pan-European Real-World (PEARL) 24-month, phase 4, prospective observational study, was initiated in 2020 to evaluate the use of fremanezumab in adult patients with EM and CM.3,4 The results of the 3rd interim analysis were presented at the European Academy of Neurology (EAN) Congress 2023, at which point all participants had completed at least 6 months of fremanezumab treatment.4 In total, 968 adult patients who had EM or CM were included in this interim analysis.4 They had a mean age of 46.5 years old and were mostly women (87.3%).4 The mean years from migraine diagnosis to fremanezumab initiation was 17.3 years and two-thirds of them had CM (n=648) vs EM (n=320).4 The majority (89.4%; n=865) of patients received the Q1M dosing with the rest having either the Q3M dosing or had switched between the two schedules at some point.4
Encouraging response with enduring real-world benefits in MMD reduction
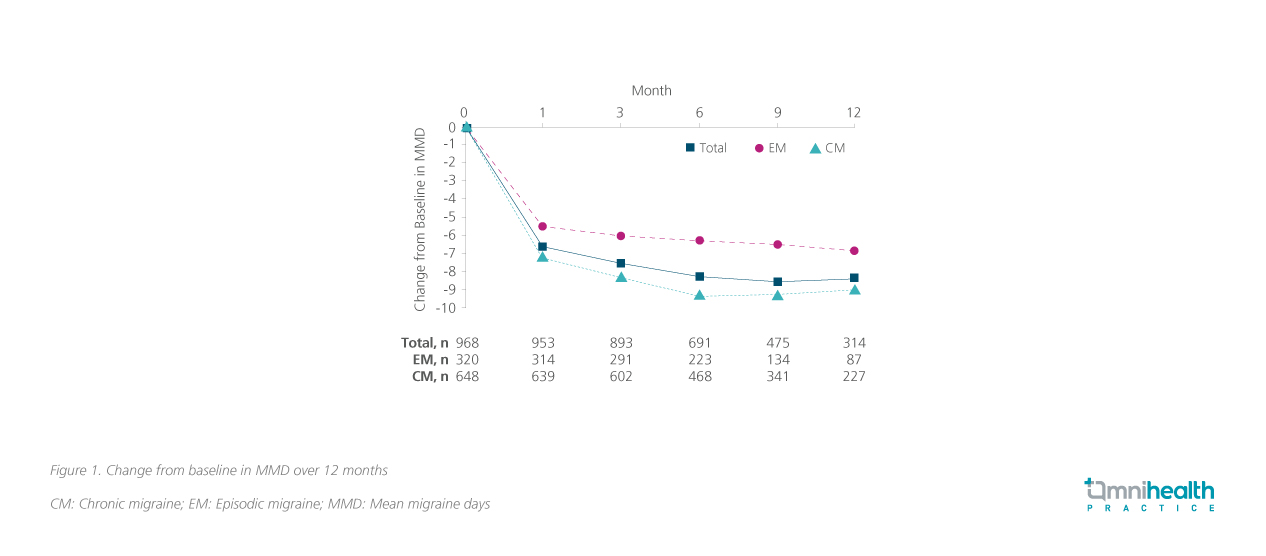
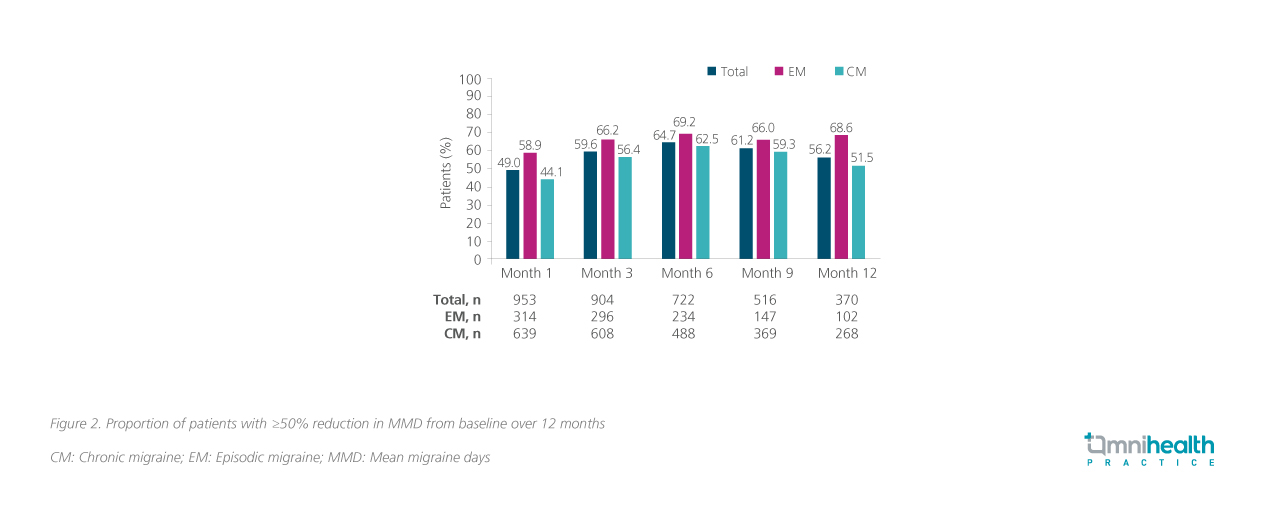
The primary endpoint of PEARL was the proportion of patients achieving a ≥50% reduction in MMD from baseline at 6 months.4 A baseline MMD of 14.6 days was established over 28 days.3,4 In the overall population, over half (58.5%; n=428) of the patients had achieved the primary efficacy endpoint.4 When stratified by migraine type, more patients with EM (67.7%; n=159/235) achieved the primary efficacy endpoint compared with the CM group (54.1%; n=269/497).4 Both the change in MMD from baseline (figure 1) and the proportion of patients achieving a ≥50% reduction in MMD from baseline (figure 2) had maintained relatively stable over 12 months.4
Only a small number of patients (n=62) had prior exposure to a CGRP pathway mAb, most had received erenumab (n=59), while one had galcanezumab, and two other patients had tried both medications.5 The mean duration of treatment was 11.6 months for erenumab and 4.0 months for galcanezumab.5 Lack of efficacy was cited as the reason for the switch in 41.9% of all cases.5 Despite treatment with prior CGRP pathway mAbs, reasonable efficacy was maintained with 32.3% of patients still achieving the primary efficacy endpoint.5 Notably, 60.9% of patients in the CM group who had switched (n=28) achieved a ≥30% reduction in MMD from baseline at 6 months which was comparable to the 75.1% of patients in the overall CM group regardless of prior medication.5
Exploratory endpoints and reduction in disability scores
Beyond the measure of MMD, the duration and severity of migraine attacks also had a significant impact on QoL.6 The mean pain numeric rating scale (NRS) score assessing severity dropped from 6.7 to 4.3 and 6.8 to 5.4 in the EM and CM cohorts respectively after 12 months of fremanezumab treatment.6 Similarly, the duration of migraine attacks was shortened at 12 months in both the EM (7.7 to 5.2 hours) and CM cohorts (8.8 to 6.5 hours) respectively.6
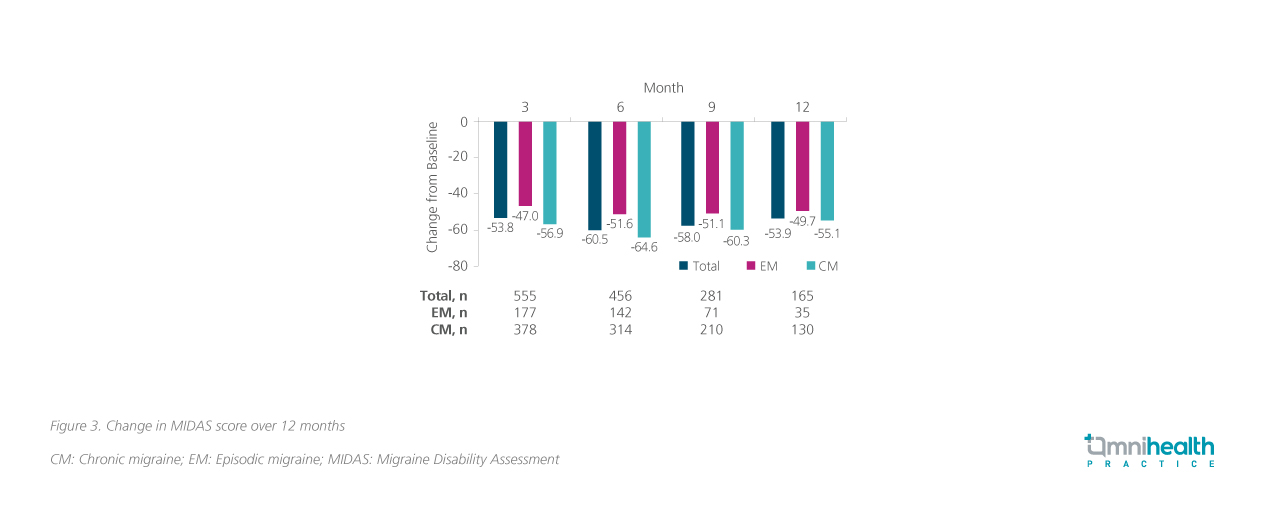
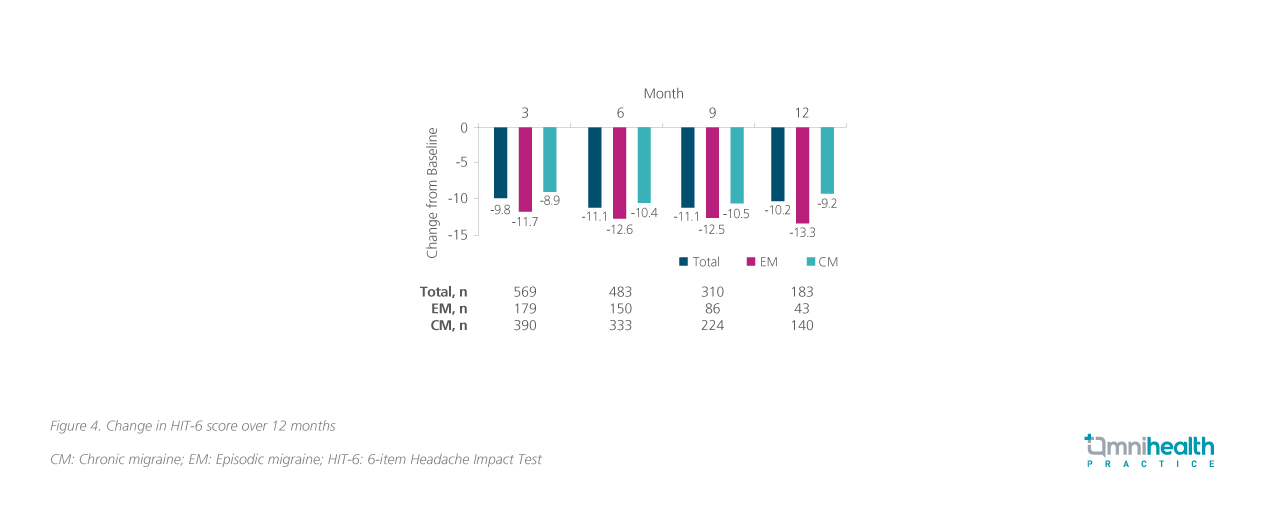
Disability outcomes were also reported in the form of the 6-item Headache Impact Test (HIT-6), and Migraine Disability Assessment (MIDAS) scores over 12 months.3 The HIT-6 is a validated questionnaire that measures the impact of headaches on social functioning, role functioning, vitality, cognitive functioning, and psychological distress, while the MIDAS quantifies the disability (by lost days of activity over 3 months) caused by headaches in the domains of work, household work, and non-work respectively.3 Consistent improvement was reported starting three months into treatment and was maintained up till 12 months across both metrics (figure 3 and 4).4
Furthermore, the use of concomitant medication (both acute and preventive) can be used as a proxy for treatment outcome.7 33.2% (n=321) patients used concomitant preventive medication with tricyclics, beta-blockers and anticonvulsants as the most common.7 However, their use has decreased since the start of fremanezumab treatment.7 Barely half (51.3%; n=497) of all patients had used concomitant acute migraine medication.7 There was a notable decrease in the mean monthly number of days with triptan use within one month of fremanezumab initiation which had persisted over time.7
Consistent safety profile with proven adherence and persistence
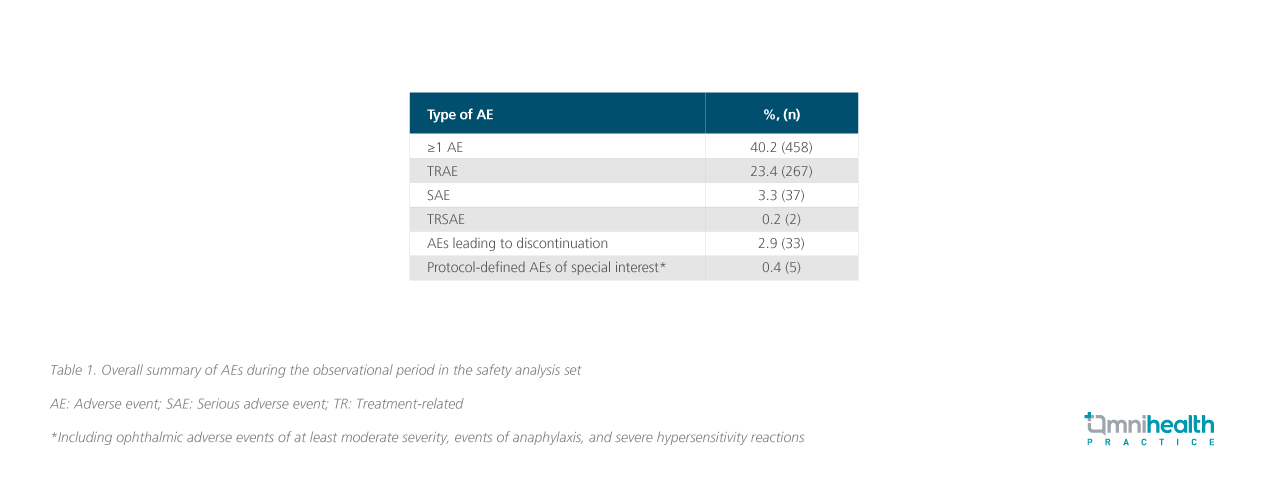
Data from the full safety analysis set (n=1,140) were also presented at this interim analysis with rates that were generally consistent with prior reports (table 1).6 The most frequent treatment-related adverse effects (TRAEs) were injection site reactions (14.9%) and gastrointestinal disorders (5.6%).6
The safety profile was also reflected in the high rates of adherence and persistence among the PEARL cohort.7 Most patients had stayed on treatment throughout the analysis period as indicated by the high rate of persistence as measured by the continued administration of fremanezumab throughout the trial.7 There was minimal decline in persistence ranging from 97.2% at 3 months to 82.3% at 12 months.7 Adherence was defined as when fremanezumab was administered within 5 days before or after the prescribed dosing schedule.7 When adherence was tabulated cumulatively after the first incidence of non-adherence regardless of subsequent administrations, there was a steady decline from 85.6% at 3 months to 41.4% at 12 months.7 However, overall adherence assessed at individual appointments regardless of prior time points was high, ranging between 91.7% and 96.5% starting from the second injection.7
Conclusion
The 3rd interim analysis of PEARL investigated the use of fremanezumab in real-world patients with either EM or CM, offering insights into how it helps reduce MMD and improve other measurements of migraine impact. 58.5% of patients achieved a ≥50% reduction in mean migraine days (MMD) from baseline at 6 months with fremanezumab, with efficacy maintained in 32.3% of the population with prior CGRP pathway mAb uses.4,5 Other relevant outcomes including reduction in severity, duration, disability, and concomitant medication use were also evident after the initiation of fremanezumab.4,6,7 These findings may better inform clinical practice and establish guidelines to optimize prescription procedures.3
Presentation: Fremanezumab for injection. Indications: For prophylaxis of migraine in adults who have at least 4 migraine days per month. Dosage and administration: Monthly dosing - 225 mg once monthly administered subcutaneously. Quarterly dosing - 675 mg every three months administered subcutaneously. When switching dosing regimens, administer the first dose of the new regimen on the next scheduled dosing date of the prior regimen. Assess treatment benefit within 3 months after treatment initiation. Recommend regular evaluation of need of treatment continuation thereafter. Resume the indicated dose as soon as possible in case of missed dose. No double dose for replenishment. Elderly - No dose adjustment is required. Renal impairment - No dose adjustment is required. Hepatic impairment - No dose adjustment is required. In case of overdose, monitor the patient for any signs or symptoms of adverse effects and given appropriate symptomatic treatment if necessary. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Discontinue administration and initiate appropriate therapy if a hypersensitivity reaction occurs. Precautions and warnings: Hypersensitivity. Major cardiovascular diseases. Pregnancy and lactation: Avoid the use of fremanezumab due to limited data on its use in pregnant women to inform a drug-associated risk. There is no information regarding the presence of fremanezumab in human milk and the effects on the breastfed infant. A decision must be made whether to continue the use of fremanezumab while breast-feeding. Undesirable effects: Injection-site reactions (pain, induration, erythema, and pruritus) For other undesirable effects, please refer to the full prescribing information. Preparation: 1 × 255 mg/1.5ml prefilled pen. Legal Classification: Part 1, First & Third Schedules Poison Full prescribing information for AJOVY upon request.
The material is for the reference and use by healthcare professionals only.
Full prescribing information for AJOVY upon request.
The material is for the reference and use by healthcare professionals only.
| Teva Pharmaceutical Hong Kong Limited Room 2303, 23/F, Mira Place Tower A, 132 Nathan Road, Tsim Sha Tsui, Kowloon, Hong Kong Tel (852) 3188 4288 Fax (852) 3585 6220 |

