CONFERENCE UPDATE: AAAAI 2024
Symptom reduction and QoL improvement in CRSwNP patients with mepolizumab: Results from the phase 3 SYNAPSE trial
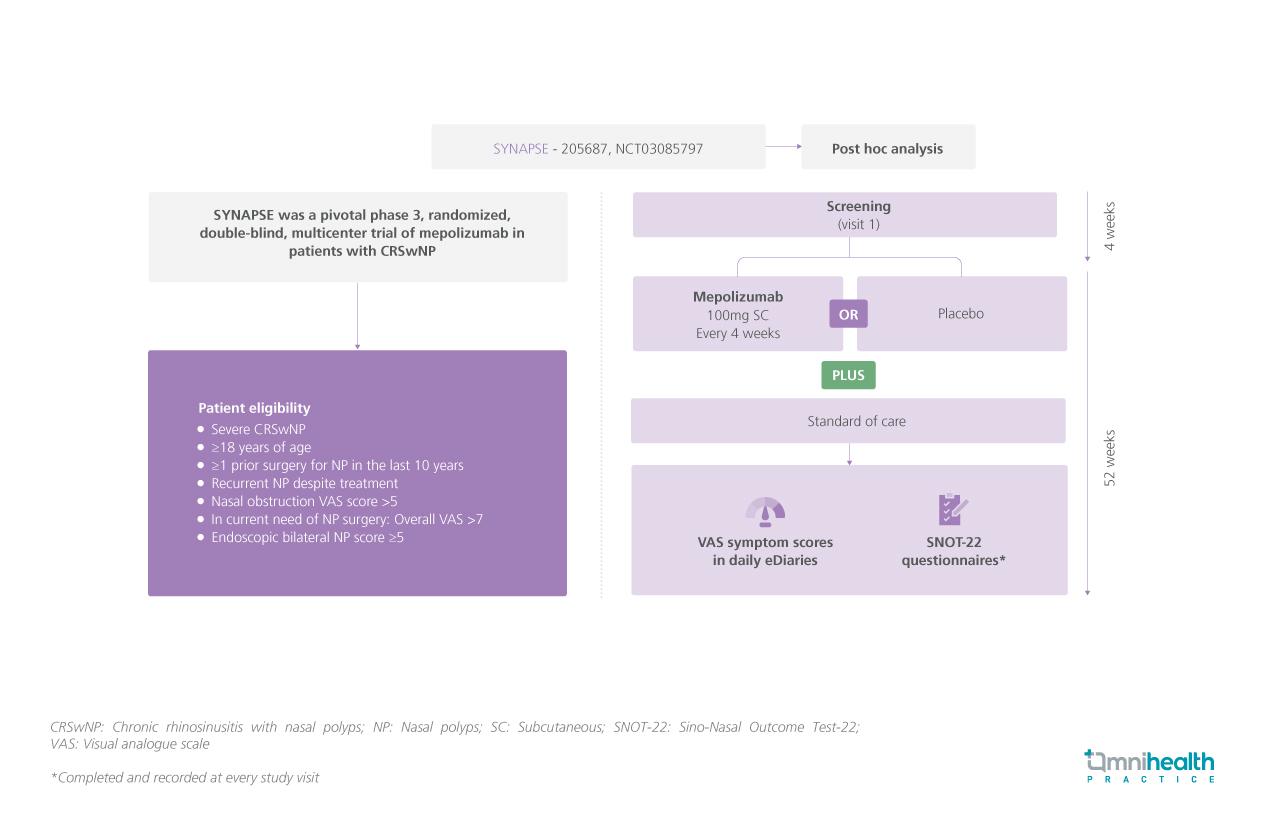
STUDY DESIGN
Chronic rhinosinusitis with nasal polyp (CRSwNP) is a chronic disease characterized by elevated interleukin 5 (IL-5) levels and is often associated with severe sinonasal symptoms and nasal obstruction.1 Mepolizumab is a humanized anti-IL-5 antibody approved as an add-on medication for CRSwNP.1 In the pivotal phase 3 SYNAPSE study, mepolizumab demonstrated improvement in sinonasal outcomes and reduction in corticosteroid use or surgery need in patients with CRSwNP.1 However, its effect on the number of low-symptom/symptom-free or symptom-reduced days remained unclear.1
SYNAPSE study was a phase 3, randomized, double-blind, multicenter trial conducted to examine the impact of mepolizumab on symptom reduction and improvements in quality of life (QoL) among patients with CRSwNP.1 Adult patients with severe CRSwNP, and had at least 1 prior surgery for nasal polyps (NP) with recurrent nasal polyps despite treatment with an endoscopic bilateral NP score ≥5.1 They also scored >7 for the current need for NP surgery and >5 for nasal obstruction on a visual analogue scale (VAS).1 They were randomized to receive mepolizumab (n=206) or placebo (n=201) plus standard of care for 52 weeks.1
The study outcomes were the presence of low-symptom or symptom-free days (defined as a VAS ≤3), symptom-reduced days (defined as VAS score ≤3 or improvement of ≥1 in VAS score from baseline), and improvements in symptom severity from severe to low/moderate [assessed by the VAS and Sino-Nasal Outcome Test-22 (SNOT-22) scores] were assessed from baseline and at weeks 49 to 52.1
FINDINGS
| Study outcomes: |
|
|
|
|
|
“Among patients with a very high burden of disease, IL-5 inhibition had a beneficial impact on clinical measures meaningful for patients’ QoL”
Dr. Larry Borish
Departments of Medicine and Microbiology,
University of Virginia Health Systems,
Charlottesville, Virginia,
United States

