CONFERENCE UPDATE: ERS 2024
Dupilumab reduces the use of OCS and antibiotics in patients with CRSwNP in real-world practice
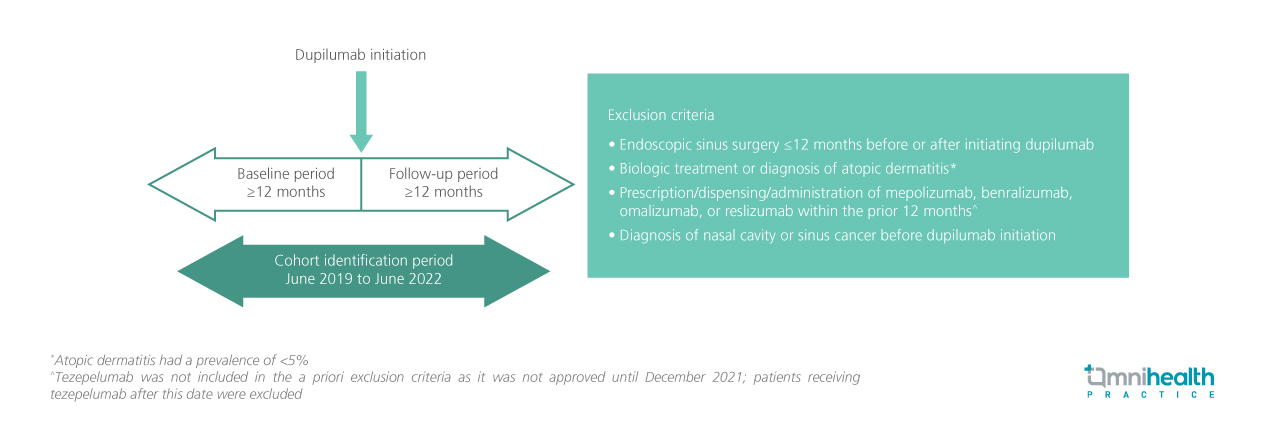
STUDY DESIGN
Dupilumab is indicated as an add-on maintenance treatment for adult patients with inadequately controlled chronic rhinosinusitis with nasal polyps (CRSwNP).1 It has been shown to improve both objective and patient-reported outcomes in this population.1 However, data on dupilumab’s effectiveness in clinical practice in the United States (US) remains limited.1 As such, the captioned study assessed the medication burden, specifically the use of oral corticosteroids (OCS) and other medications, in CRSwNP patients before and after initiating dupilumab in real-world US settings.1
This was a retrospective observational cohort study of adult patients with CRSwNP who initiated dupilumab 300mg every two weeks between June 2019 and June 2022.1 OCS and antibiotic use data were drawn from the OM1 Real-World Data Cloud and the Reg-ENTSM Registry.1 Patients were excluded if they had had endoscopic sinus surgery within 12 months before or after dupilumab initiation, prior biologic treatments, or diagnoses of atopic dermatitis.1 Patients prescribed, dispensed, or administered with, mepolizumab, benralizumab, omalizumab, or reslizumab within the previous 12 months, or diagnosed with nasal cavity or sinus malignancy before dupilumab initiation, were also excluded.1 The cohort included 1,016 patients, of whom 724 (71.3%) had a history of allergic rhinitis, and 579 (57.0%) had a history of asthma.1
The analysis summarized CRSwNP-related OCS use (defined as OCS use within 5 days of a chronic rhinosinusitis [CRS]/nasal polys [NP] diagnosis or within 30 days of sinus surgery), OCS bursts (defined as steroids use within a duration of ≤14 days with ≥7 days between bursts), and the prescription of OCS and antibiotics in the 12 months before and after dupilumab initiation (pre-/post-dupilumab periods)
FINDINGS
|
Outcomes: |
|
The analysis summarized CRSwNP-related OCS use, OCS bursts and the prescription of OCS and antibiotics in the 12 months before and after dupilumab initiation (pre-/post-dupilumab periods)1 |
|
The proportion of patients with CRSwNP-related OCS use decreased from 59.1% pre-dupilumab to 17.7% post-dupilumab1 |
|
Overall OCS use decreased from 68.1% pre-dupilumab to 28.7% post-dupilumab (p<0.001)1 |
|
Chronic CRSwNP-related OCS use (defined as >30 cumulative days of supply) decreased from 3.3% pre-dupilumab to 0.7% post-dupilumab1 |
|
Chronic CRSwNP-related OCS bursts (≤14 days with ≥7 days gap between bursts) pre-dupilumab was 31.1% (mean [SD]=1.5 [0.9] per patient) vs. 14.1% post-dupilumab (mean [SD]=1.2 [0.5] per patient)1 |
|
76.5% of patients with CRSwNP-related OCS use pre-dupilumab did not require OCS post-dupilumab1 |
|
Antibiotic use decreased from 64.6% pre-dupilumab to 31.8% post-dupilumab1 |
"Patients with CRSwNP who initiated dupilumab had reduced antibiotic and corticosteroid use during the 12 months following initiation compared with the 12 months pre-dupilumab"
Dr. Stella E. Lee
Brigham and Women’s Hospital,
Harvard Medical School,
Boston, United States

