CONFERENCE UPDATE: 2025 AAAAI/WAO Joint Congress
Tezepelumab’s impact on activity levels in patients with severe, uncontrolled asthma and CRSwNP: Post-hoc analysis of the phase 3 NAVIGATOR study
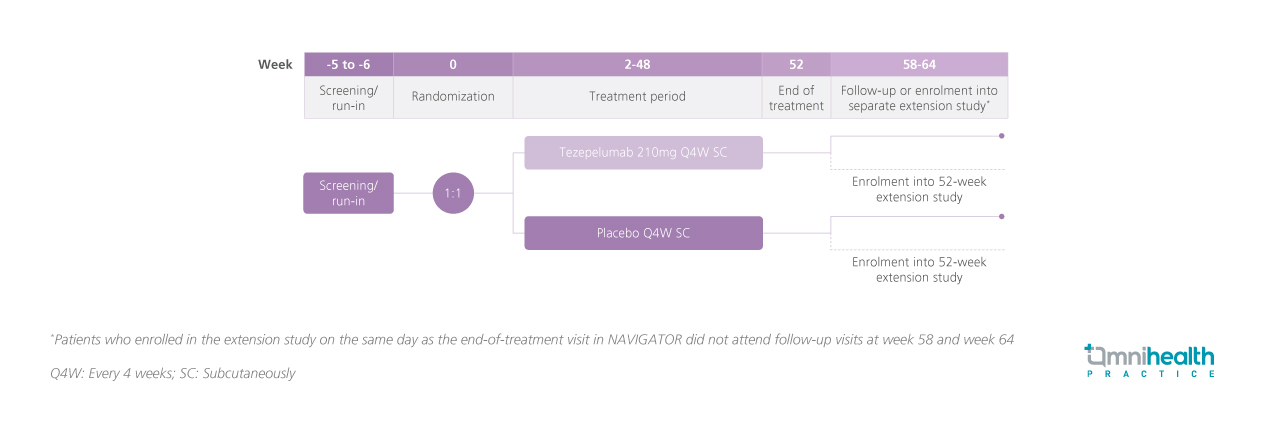
STUDY DESIGN
Severe asthma and chronic rhinosinusitis with nasal polyps (CRSwNP) can significantly limit patients' activity levels.1 This post hoc analysis of the phase 3 NAVIGATOR study assessed the impact of tezepelumab on activity-related items within the Asthma Quality of Life Questionnaire (standardized) for patients aged ≥12 years (AQLQ(S)+12) who have a history of comorbid CRSwNP.1 In the NAVIGATOR study, patients aged 12-80 years with severe, uncontrolled asthma were randomized to receive either tezepelumab 210mg (n=528) or placebo (n=531) via subcutaneous injection every 4 weeks (Q4W) for 52 weeks.1 A total of 90 and 75 patients had a history of comorbid CRSwNP in the tezepelumab and placebo arms, respectively.1 The AQLQ(S)+12 was administered at baseline and at week 52, to evaluate changes in activity-related limitations.1 Patients were classified as responders if they had an initial score of 1-5 at baseline (1: severe limitation; 5: some limitation) and improved to a score of 6-7 at week 52 (6: little limitation; 7: no limitation).1 Among those assessed, 77 patients in the tezepelumab group and 55 patients in the placebo group completed the AQLQ(S)+12.1 Outcomes of interest in the analysis included improvement in individual activity-related items within the AQLQ(S)+12 after 52 weeks of treatment.1
"The patients with severe, uncontrolled asthma and a history of comorbid CRSwNP who received tezepelumab showed substantial improvements in activity levels versus placebo recipients"
Dr. Anju Peters
Division of Allergy and Immunology,
Feinberg School of Medicine,
Northwestern University,
Evanston, United States
FINDINGS
|
Efficacy outcomes: |
|
|
|

