CONFERENCE UPDATE: ESC 2023
4-year edoxaban treatment revealed to be well-tolerated among patients with non-valvular AF: The ETNA-AF-Europe study
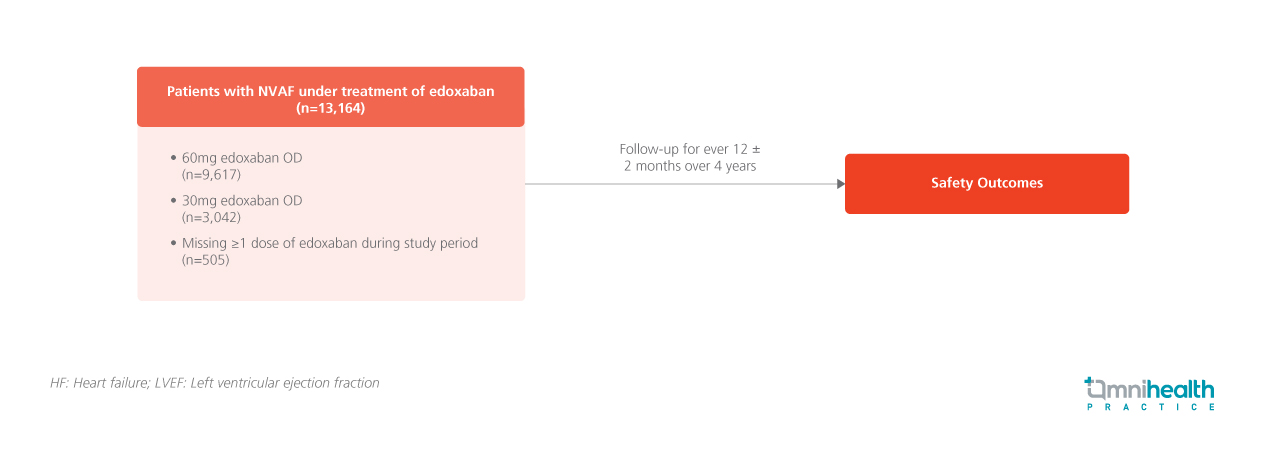
STUDY DESIGN
Non-vitamin K antagonist oral anticoagulants (NOACs) are currently the first-line medications in stroke prevention for patients with atrial fibrillation (AF).1 However, long-term non-interventional data in patients with AF is limited.1 The ETNA-AF-Europe study was a prospective, observational, multicentre safety study, designed to assess the safety profile of edoxaban in patients with non-valvular AF (NVAF) in the real-world setting.1
A total of 13,164 patients with NVAF were enrolled in this study, of which 73.1% received 60mg of edoxaban once daily (OD), 23.1% of patients received 30mg of edoxaban OD and 3.8% of patients had missed their doses during the study period.1 The cohort which received 30mg had a lower creatinine clearance rate, a higher median age, higher proportion of frailty, and higher Congestive heart failure, Hypertension, Age, Diabetes, previous Stroke/transient ischemic attack, Vascular disease and Sex category (CHA2DS2-VASc) score.1 Throughout the 4 years of study duration, patients were followed up every 12 ± 2 months, enabling long-term monitoring of real-world scenarios on edoxaban use.1 The primary endpoints of this study consisted of the annualized incidence rate of all-cause death, cardiovascular (CV) death, intracranial hemorrhage (ICH), major bleeding, and major gastrointestinal (GI) bleeding events.1 Secondary endpoints include the annualized incidence rate of stroke, transient ischemic attack (TIA), and systemic embolic event (SEE).1
The endpoints of this study were the annualized incidence rates of all-cause and CV death, ICH, major bleeding and major GI bleeding.1 Secondary endpoints include the annualized incidence rate of stroke, TIA and SEE1
| Primary endpoint: |
|
|
|
|
|
| Secondary endpoints: |
|
|
|
"The 4-year follow-up data provided robust evidence for the clinical effectiveness and safety of edoxaban in patients with AF."
Dr. Raffaele De Caterina
Cardiology Division,
University Hospital of Pisa,
Pisa, Italy

