MEETING HIGHLIGHT
Emerging from covid-19 predicament: An update on its management and vaccination
The coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has afflicted more than 200 million people, with a death toll of more than 4 million people worldwide.1 As of September 29, 2021, Hong Kong has recorded 12,210 COVID-19 cases with 213 deaths.2 Although the situation in Hong Kong has been stabilized since the end of the fourth wave of epidemic in May 2021, the emergence of variants, especially the Delta variants, could be another threat to our society.3,4 In a lecture held by the Macau Association of Health Service Executives (MAHSE), Professor Hung, Fan-Ngai Ivan addressed the concerns over the emergence of the Delta variant and provided an update on the COVID-19 management and vaccination.
COVID-19 disease pathogenesis
A large cohort study revealed that up to 81% of COVID-19 patients had mild-to-moderate disease and, around 19% had severe-to-critical conditions.5 In patients with mild-to-moderate disease, the immune response triggered by SARS-CoV-2 was self-limited, and recovery could usually be achieved within 3 weeks since the onset of symptoms (Figure 1).6 Some patients who have recovered may have detectable SARS-CoV-2 ribonucleic acid (RNA) in their upper respiratory specimens for up to 3 months, but it does not necessarily indicate replication-competent virus (i.e., infectiousness) or the presence of a new infectious virus.5
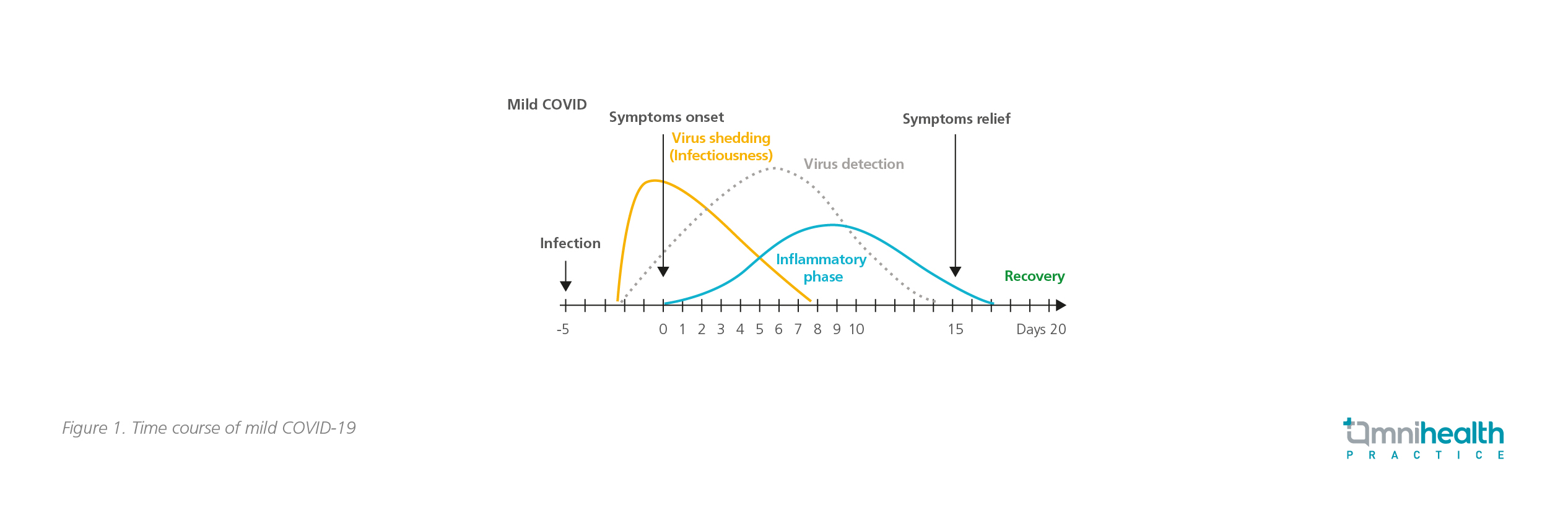
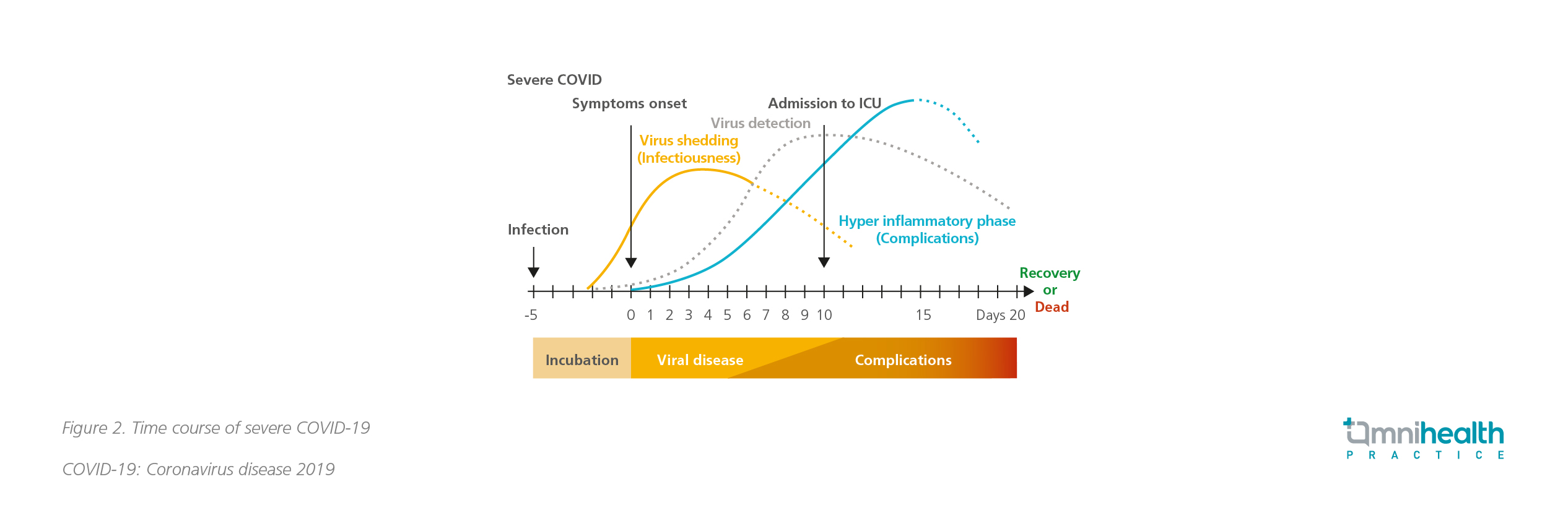
Severe COVID-19 cases were characterized by a unique and inappropriate inflammatory response, with low levels of type I and III interferons and increased level of interleukin-6 (IL-6), subsequently resulting in damages to the pulmonary and cardiovascular systems (Figure 2).6,7 Evidence showed that the risk of developing severe disease and death from COVID-19 significantly increased with advancing age and was the highest among the oldest cohort.5 Limited data also suggested that severely immunocompromized patients, such as those with lymphoma, organ transplant and acquired immunodeficiency syndrome (AIDS), might have prolonged exposure to replication component virus beyond 20 days and are more likely to get severe diseases.5,8
 Treating severe COVID-19 cases
Treating severe COVID-19 cases
Patients with severe COVID-19 disease required hospitalization. The inpatient management includes supportive care for pneumonia, hypoxemic respiratory failure, septic shock, cardiomyopathy and arrhythmia, acute kidney injury, and thromboembolism.5 “The key to prevent complications and reduce mortality among severely ill COVID-19 patients is to treat early,” stressed Prof. Hung. The current treatment options include antiviral agents, interferons and neutralizing antibodies. Nevertheless, in order to prevent the emergence of viral resistance and achieve rapid suppression of viral replication, combinations of these therapeutic agents are often required.
Triple combination strategy
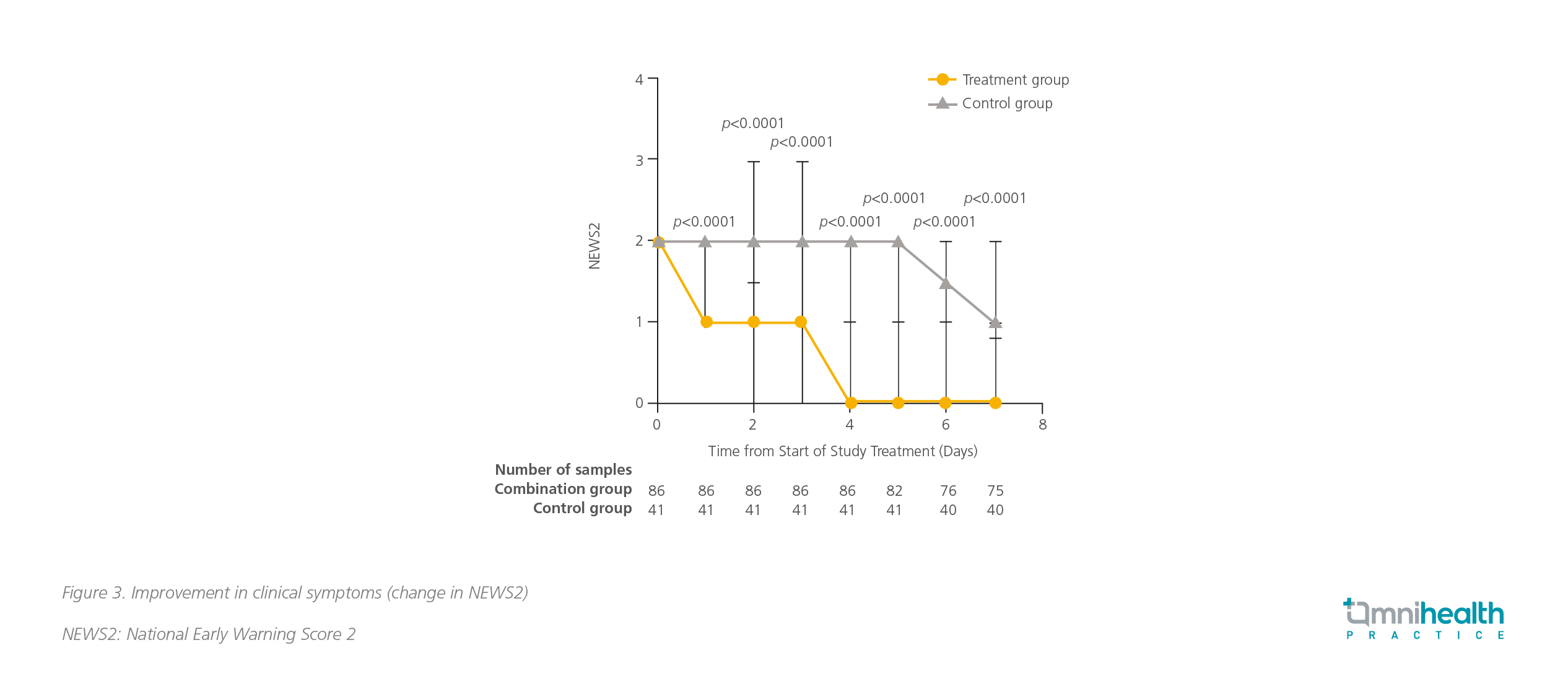
The result of a phase 2 study in Hong Kong demonstrated that early triple combination of interferon beta-1b, lopinavir-ritonavir and ribavirin has shown a more superior efficacy than using lopinavir-ritonavir alone in alleviating symptoms and shortening the duration of viral shedding and hospitalization in patients with mild-to-moderate COVID-19.9 Of note, all patients had an SARS-Cov-2-positive baseline nasopharyngeal swab.9 Patients on triple therapy had a significantly shorter median time to achieve negative result in nasopharyngeal swab than the patients in the control group [7 days vs. 12 days; HR=4.37 (95% CI: 1.86-10.24), p=0.0010].9 Clinical improvement was also significantly better in the combination group.9 The time to complete alleviation of symptoms, defined as National Early Warning Score 2 (NEW2), was 4 days in the treatment group versus 8 days in the control group [HR=3.92 (95% CI: 1.66-9.23), p<0.0001] (Figure 3).9
Dual-antibodies therapy
Casirivimab and imdevimab are 2 monoclonal antibodies extracted from humanized mice and peripheral B-cells of patients recovered from COVID-19.10 They prevent SARS-CoV-2 from invading human host cells by uncompetitively binding to 2 different sites on the receptor-binding domain (RBD) of the viral spike protein.10 This combination therapy retains neutralization potency against circulating SARS-CoV-2 variants of concern, including B.1.1.7 (or alpha), B.1.351 (or beta), B.1.617.2 (or delta), B.1.429 (or epsilon), and P.1 (or gamma).10 This antibody combination has been granted the Emergency Use Authorization (EUA) in non-hospitalized mild-to-moderate COVID-19 patients in the United States (US), and authorized by the European Medicines Agency (EMA) for use in patients who are at high risk of advancing to severe COVID-19 but do not require supplemental oxygen.10 Clinical trials are ongoing to evaluate its efficacy in hospitalized patients. The interim result of the ongoing RECOVERY trial demonstrated that this combination therapy achieved a significant reduction of 20% in 28 day-mortality, with an absolute benefit of 6 fewer deaths per 100 hospitalized seronegative patients.10 The rate of progression to invasive mechanical ventilation or death in these patients was also reduced.10
Prevention is better than cure
Prevention is always better than cure. Apart from maintaining good personal hygiene and social distancing, vaccination against SARS-Cov-2 is also vital. Nowadays, there are more than 100 vaccines in clinical trials around the world, including an intranasal vaccine developed by the University of Hong Kong.11,12 About 20 vaccines have been approved by the health authorities for limited or full use globally.11 BNT162b2 and CZ02 are two of the approved vaccines and currently being used for vaccination in Hong Kong.
Positive efficacy and safety data of the vaccines
BNT162b2 is nucleoside-modified messenger ribonucleic acid (mRNA) vaccine. The mRNA encodes the viral spike glycoprotein of SARS-CoV-2. Upon the entry of mRNA into host cells, it allows the expression of the spike antigen, thus eliciting the immune response against SARS-CoV-2.13 A phase 3 study showed that a 2-dose regimen of BNT162b2 conferred 95% protection (95% CI: 90.3-97.6) against COVID-19 in patients aged 16 years and above.14 Similar vaccine efficacy was observed across the subgroups defined by age, sex, race, baseline body-mass index, and the presence of coexisting conditions.14 The interim result of an ongoing study revealed that the vaccine was also safe and efficacious in 12- to -15-year-old adolescents, producing an even greater immune response than in young adults.15 Besides, CZ02 is an inactivated whole-virion SARS-CoV-2 vaccine.16 The interim result of a phase 3 study in Turkey demonstrated that a 2-dose regimen of CZ02 yielded a vaccine efficacy of 83.5% (95% CI: 65.4-92.1).16 Both BNT162b2 and CZ02 demonstrated the favourable safety profile and were well-tolerated. Most reported adverse events (AEs) were short-term, mild-to-moderate pain at the injection site, fatigue and headache.14,16
A study evaluating the serological response to the mRNA and inactivated COVID-19 vaccines in Hong Kong healthcare workers was performed in April 2021.17 The preliminary results showed that the majority of the participants developed positive anti-spike immunoglobulin G (IgG) after receiving 2 doses of BNT162b2 or CZ02 without significant difference between the 2 arms (100% vs. 99%, p=1).17 Of note, the IgG and total antibody levels induced by BNT162b2 were higher than CZ02.17 In addition, more BNT162b2 recipients had positive surrogate neutralizing antibody results (100% vs. 94%, p<0.0194).17
Emergence of Delta variant: A looming threat to Hong Kong
Variants of SARS-CoV-2 have emerged, spreading around the world since the beginning of the COVID-19 pandemic.18 Currently, there are more than 10 variants, including alpha and beta variants, being monitored by the US Centers for Diseases Control and Prevention (CDC) and the World Health Organization (WHO).18 Of particular concern is the Delta variant, the predominant variant in the US that is more contagious than the wild type of SARS-CoV-2, and is classified by CDC as the Variant of Concern (VOC).18 Some data suggested that the Delta variant could cause more severe illness than previous strains in unvaccinated people and was even able to infect those fully vaccinated people (i.e., breakthrough infection).18
Effectiveness of the vaccines against Delta variant
Public concerns over the effectiveness of the existing vaccines have been heightened. A recent study showed that a single dose of BNT162b2 only yielded protection of 30.7% (95% CI: 25.2-35.7) against the Delta variant. 19 However, the vaccine effectiveness (VE) reached 88% (95% CI: 85.3 to 90.1) when 2 doses were given.19 On the other hand, the efficacy of CZ02 against the Delta variant was demonstrated by a real-world study in Guangzhou: a single dose of the inactivated vaccine yielded the vaccine effectiveness of only 13.8% (95% CI: -60.2 to 54.8), but a 2-dose regimen conferred protection of 59% (95% CI: 16.0-81.6), exceeding the WHO’s minimal threshold of 50%.20 In summary, only modest differences in VE of the 2 vaccines were noted with the Delta variant compared with the wild type of SARS-CoV-2 after receiving 2 vaccine doses. The existing vaccines are still effective against COVID-19 caused by the Delta variant.
Protection in vaccine breakthrough infection
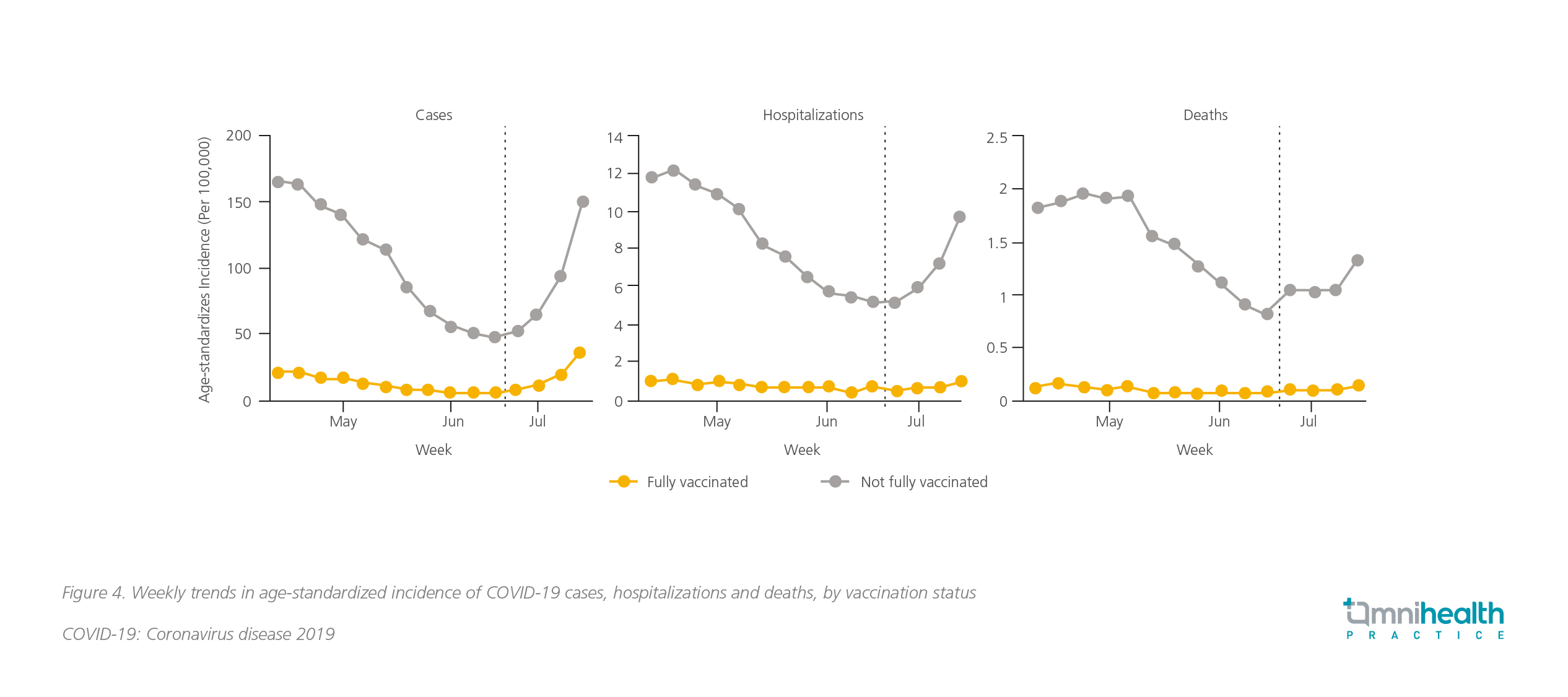
The SARS-CoV-2 Delta variant has become predominant in the US since June 2021. The COVID-19 cases have rebounded since then, even with reported cases of patients who have completed 2-doses regimen.21 Nevertheless, the US COVID-19 vaccine breakthrough infection surveillance data showed that the infection rate among fully vaccinated persons was significantly lower than those unvaccinated or not fully vaccinated (8% vs. 92%, Figure 4).21 Besides, the rates of hospitalization and COVID-19-associated deaths were reported to remain unchanged among fully vaccinated people, while the rate increased among people not fully vaccinated (Figure 4) .21
Addressing concerns over the safety of mRNA vaccines
Since April 2021, there have been reports of myocarditis or pericarditis in Hong Kong, especially in adolescents and young adults, after receiving the mRNA vaccine.22 Although most of the cases were mild requiring minimal treatment to achieve full recovery within a few days, the reports still aroused public concerns over the safety of mRNA vaccines.22 In fact, myocarditis and pericarditis are rare AEs which have also been observed in other types of vaccinations such as smallpox vaccines, with reported incidence rate of 12 to 16.1 cases per million vaccinated individuals.22 A recent dataset from CDC revealed that the myocarditis reporting rates for the mRNA vaccines were 40.6 cases per million second doses in males aged 12-29 years and 2.4 cases per million second doses in males aged ≥30 years.23 The reporting rates among females in these age groups were 4.2 and 1.0 per million second doses, respectively.23 Although these AEs are uncommon, individuals are still advised to refrain from strenuous activities for 1 week after the COVID-19 mRNA vaccination.22 Vaccinees should seek medical assistance if they experience chest pain, shortness of breath and palpitations.22
Conclusion
Elderly people are at significantly higher risk of developing severe COVID-19 and death from the associated complications. Although more treatment options are currently available for managing severe COVID-19, prevention is better than cure. Immunization with COVID-19 vaccines, especially among young people, can reduce infection with SARS-CoV-2 in the first place and is crucial to protecting the high-risk population. Despite public concerns over reduced efficacy of the existing vaccines against the emerging Delta variant, evidence showed that the effectiveness reduction was slight, and the vaccines could confer strong protection in fully vaccinated people against breakthrough infection. Besides, cases of myocarditis and pericarditis, particularly in young individuals, had been reported with the mRNA vaccine, but the incidence rate was low, and the cases were mostly mild. All in all, the benefits of vaccination still outweigh the risks of SARS-CoV-2 infection. Therefore, all eligible people, including those with stable chronic illness, are advised to receive the 2-dose COVID-19 vaccines unless there is known hypersensitivity reaction or acute medical condition. Through concerted efforts, herd immunity can thus be achieved, and it is believed that resumption of normal life in Hong Kong is just a matter of time.

