RESEARCH SPOTLIGHT
Latest updates: Six-month safety and efficacy of BNT162b2 mRNA COVID-19 vaccine
In brief
With up to 6 months of follow-up in an ongoing, placebo-controlled, observer-blinded, multinational, pivotal efficacy study, the 6-month safety and efficacy data of BNT162b2 mRNA COVID-19 have been updated and summarized in this report.1
Background
The current outbreak of the novel coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) discovered in January 2020, has spread to countries around the world with recent estimates of more than 200 million cases diagnosed and over 4 million deaths.1 Coronaviruses are enveloped, positive-sense single‐stranded ribonucleic acid (RNA) viruses, and SARS-CoV-2 belongs to the B lineage of the beta-coronaviruses.2 The predominant clinical manifestations of SARS-CoV-2 include fever, cough, and other symptoms and signs of respiratory tract infections.3,4
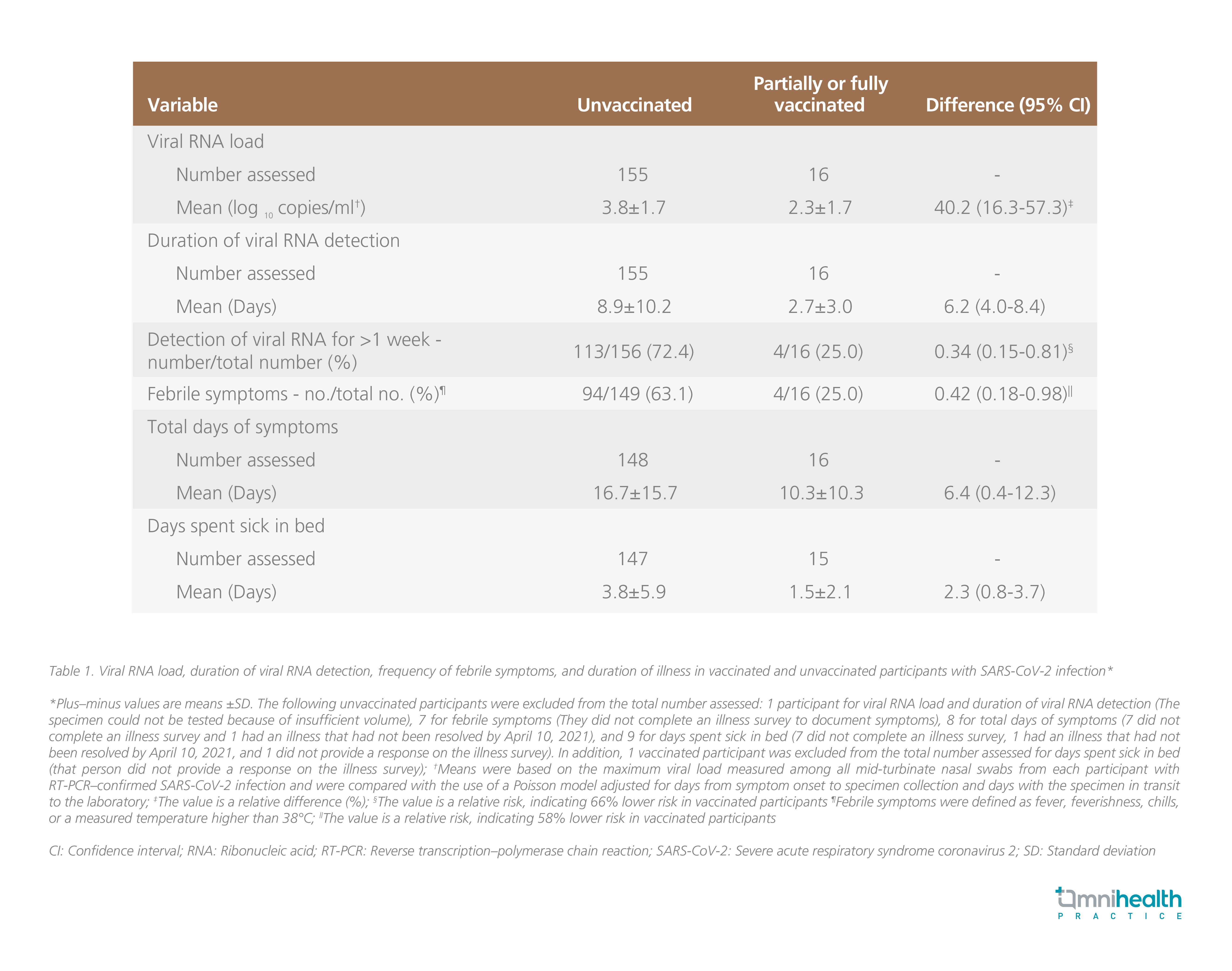
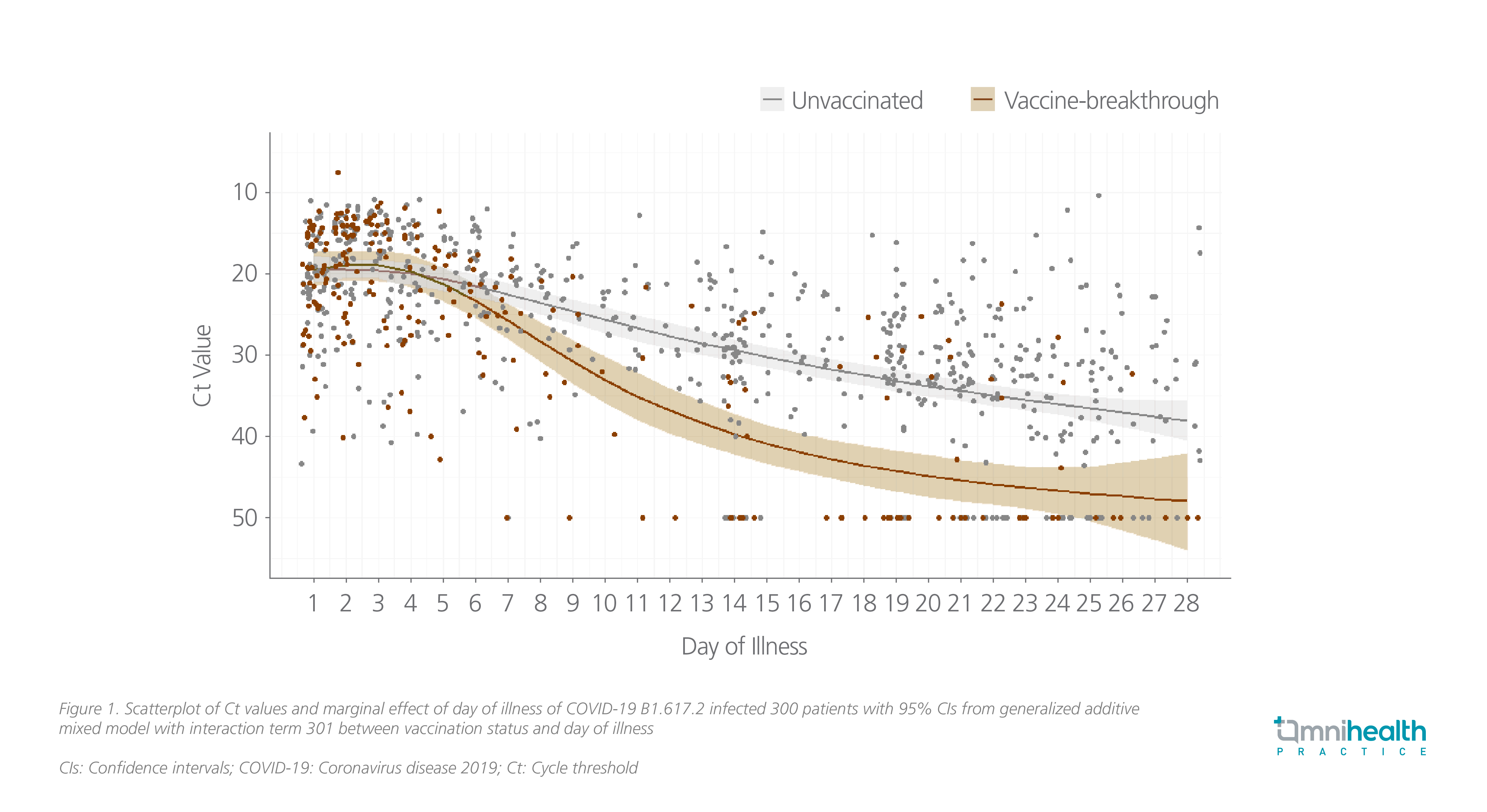
To date, billions of vaccines have been administrated worldwide. As one of the World Health Organization’s (WHO) interim recommended vaccines, BNT162b2 is a lipid nanoparticle (LNP)-formulated, nucleoside-modified RNA vaccine that encodes prefusion stabilized, membrane-anchored SARS-CoV-2 full-length spike protein.1 Upon the administration of BNT162b2, LNPs, which encapsulate and protect the naked messenger RNA (mRNA), enable the entry of mRNA into host cells.5 Once the mRNA is inside the host cells, the cells deploy their genetic machinery to translate the genetic instructions from mRNA and produce the antigens and spike proteins, which are then recognized by the immune system, on the cell surface.5 Hence, a potent immune response is produced, including antigen presentation to helper T-cells and the production of a specific type of antibodies targeting spike protein.5 As such, in the real-world data, the two-dose mRNA vaccines have shown to be highly effective in preventing SARS-CoV-2 with attenuated viral RNA load, lowering the risk of febrile symptoms, and shortening the duration of illness among those who had breakthrough infection despite vaccination (Table 1).6 In another cohort study, rapid decline in viral load and hence the transmissibility associated with mRNA vaccines was also observed among patients infected with the Delta variant (Figure 1).7
Starting from December 2020, BNT162b2 vaccine has become available for vaccine recipients at or over 16-year-olds under emergency or conditional use authorizations, yielding a 95% vaccine efficacy (VE) against COVID-19 and a favorable safety profile in diverse populations at aged 16-year-olds or above through a median of 2 months of post-immunization.4,8 As such, there is a need to assess the 6-month safety and efficacy data of BNT162b2 vaccine from vaccine recipients at or over 16-year-olds, as well as the safety, efficacy and immunogenicity data from 12 to 15-year-olds vaccine recipients.4
Methodology
To assess the safety, tolerability, efficacy, and immunogenicity of BNT162b2 in adolescents and adults, an ongoing, randomized, placebo-controlled, observer-blinded, multinational, pivotal phase 1/2/3 study was conducted.4 Currently, this report focuses on phase 2/3 safety assessments at age of 16 years or above, and prespecified VE assessments at or over 12-year-olds through up to 6 months post-immunization.1
This study enrolled participants who were healthy or of stable chronic medical conditions, and excluded patients who were in an active immunocompromising condition or having immunosuppressive therapies.1 In this study, participants were randomized 1:1 by an interactive web-based system at 152 sites, including 130 sites in the United States (US), 9 sites in Turkey, 6 sites in Germany, 4 sites in South Africa, 2 sites in Brazil, and 1 site in Argentina, to receive 2 intramuscular injections 21 days apart of 30μg BNT162b2 (0.3mL volume per dose) or saline placebo.1 Of these participants, 44,060 received 1 or more than 1 dose and 98% of them were vaccinated with 2 doses. Of participants who were vaccinated with 1 or more than 1 dose, 22,030 received BNT162b2; and 22,030 received placebo.1 In the cohort of 12 to 15-year-olds, participants were randomized across 29 US sites and 2,260 of them were vaccinated with 1 or more than 1 dose, with 1,131 receiving BNT162b2 and 1,129 receiving placebo.1 Cumulative safety follow-up was available up to 6 months post-dose 2 from combined blinded and open-label periods for 12,006 participants originally randomized to BNT162b2. Safety data and efficacy analyses have been updated till March 13, 2021 and summarized in this report.1
Results
It is remarkable that BNT162b2 continued to be well-tolerated with few cases of study withdrawal due to adverse events (AEs).1 Through up to 6 months of follow-up among vaccine recipients, 91% (95% CI: 88.8-93.0) VE against COVID-19 were reported with only 81 COVID-19 cases observed from 7 days post-dose 2, irrespective of previous SARS-CoV-2 infection.1 In addition, VE against severe disease was 97% (95% CI: 80.3-99.9).1 Notably, 100% (95% CI: 53.5-100.0) VE was seen in South Africa where the SARS-CoV-2 variant B.1.351 (beta) was predominant.1 This finding, along with all the available evidence including the real-world effectiveness data, alleviates theoretical concerns over potential vaccine-mediated disease enhancement.1
Significant interpretation and discussion
This 6-month study demonstrated that BNT162b2 was highly efficacious in preventing COVID-19 for up to 6 months.1 Dr. Wilson Lam explained that numerous factors may contribute to this triumph in durability, including the novel vaccine platform, the production time, ages of recipients, the predominance of variants, to list but a few. Among these possible factors, Dr. Lam stated that the vaccine platform should be the main determinant for the high VE in the study, as non-live vaccines usually have much lower efficacies. Dr. Lam admitted that prevention and protection against host transmission and severe disease would be the most important parameters to assess the protection of a vaccine. Apart from this, reduction of adverse outcomes is another important parameter due to the fact that the COVID-19 pandemic would probably last for a few years at least in Hong Kong and worldwide and reducing severe disease by vaccine administration would ease the burden on the healthcare system. Durability is another parameter, which is also one of this study’s major objectives. The higher the durability, the longer the duration that the vaccine recipients contribute to herd immunity. Dr. Lam asserted that vaccines of short durability may increase the likelihood of the emergence of new variants.
Even though a great VE was observed in the 6-month duration of follow-up period, it is still insufficient to demonstrate the long-term efficacy of COVID-19 vaccines. Given the fact that antibody levels often decline 6 months after the vaccination, and that efficacy would be affected, Dr. Lam emphasized the importance of a longer follow-up period which can further assess the durability of this novel vaccine and help the authority alter the vaccine strategies, such as the necessity of a booster dose.
Dr. Lam shared his experience that mild-to-moderate injection site pain and systemic events including fatigue are common in Hong Kong, while low-grade fever is less common but is still observed. Members of the public should be aware of these side effects which may occur after vaccination. Meanwhile, people may have a misconception that the side effects have a correlation with low VE and effectiveness. This should be clarified that extrinsic factors like vaccine composition, and intrinsic factors like host characteristics can affect the reactogenicity profile of a vaccine. Thus, AEs vary from individual to individual, and there is no necessary association between adverse reactions and the VE. Besides, Dr. Lam recommended that medicines should only be taken when prominent side effects occur, but not as a precautionary measure. He explained that one should not take medicine without the occurrence of side effects because it is uncertain that some anti-inflammatory drugs for alleviating fever may cause a possible, diminished immune response to vaccines.
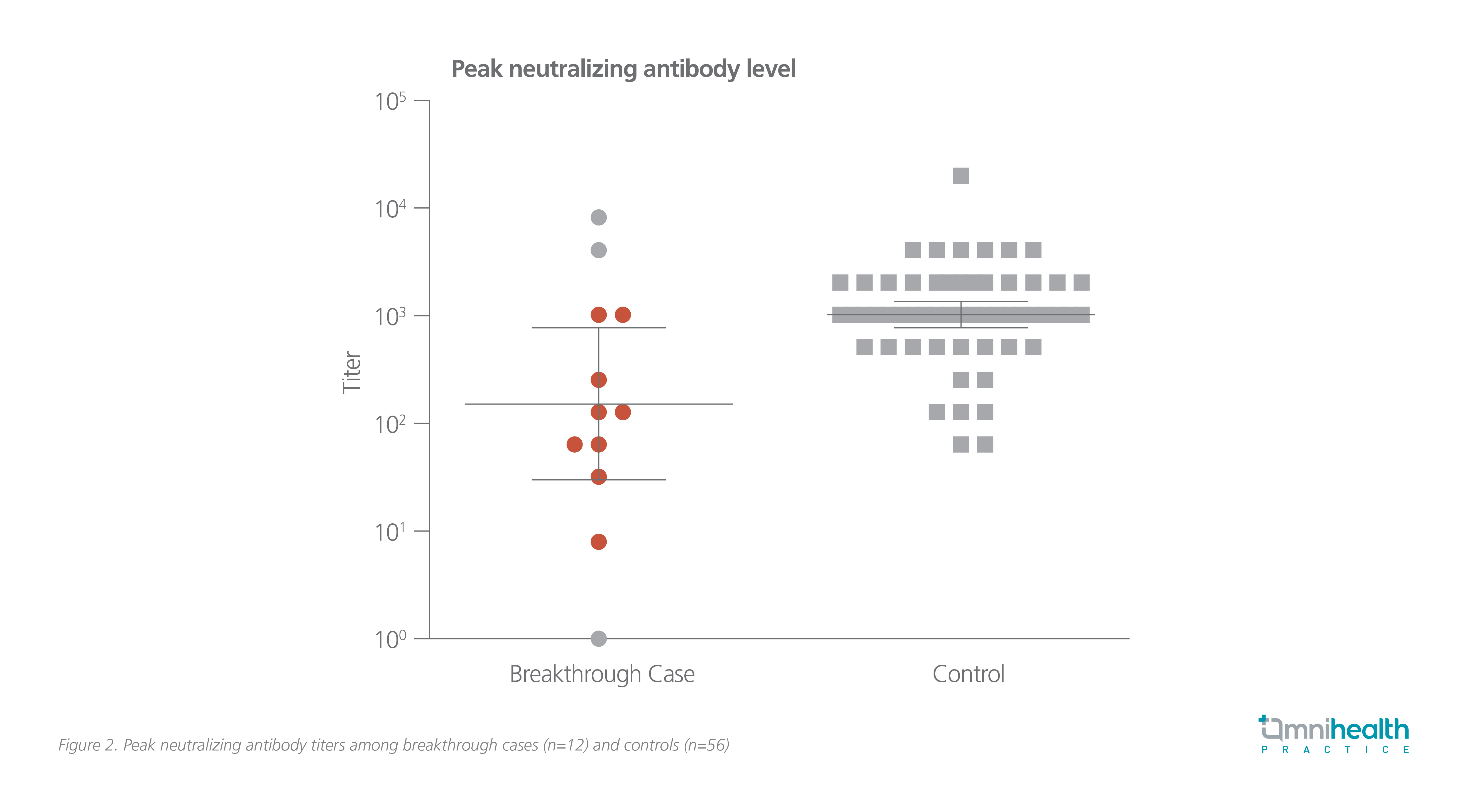
For many people who are concerned about the breakthrough infection, Dr. Lam stressed that so far there has been no local case, and that only people who are frequently exposed to the virus are prone to breakthrough infections. Thus, the VE has been promising. Dr. Lam supplemented that the selection of vaccines may affect the chance of getting breakthrough infections to a certain degree since studies have revealed an enormous difference in the neutralizing antibody titers between the vaccine platforms. The correlation between the occurrence of breakthrough infections and neutralizing antibody titers was suggested in a study in Israel involving 1,497 fully vaccinated healthcare workers.3 In the study, a lower generalized estimating equation (GEE) which predicted peak neutralizing antibody titer was recorded in the breakthrough cases (152.2; 95% CI: 30.5-759.3) versus controls (1,027.5; 95% CI: 761.6-1386.2) for a ratio of 0.148 (95% CI: 0.040-0.548), supporting the correlation between the presence of neutralizing antibodies and protection from infection with SARS-CoV-2 (Figure 2).3 In short, the neutralization level is highly predictive of immune protection, with high neutralizing antibody titers representing lower infectivity.3
Dr. Lam reminded that no matter which types of vaccines have been chosen, medical surveillance is still needed, particularly for the population group at risk, including healthcare workers and those who work in the international ports. Besides, in view of the current variant infections trend worldwide, Dr. Lam affirmed that the emergence of and rapid dominance by variants are the main causes of breakthrough infections.
With the advocacy of the nearly “zero local infections”, it is essential to keep a close eye on the presence of variants in the imported cases. Among the major 4 variants emerging since late 2020, the Delta variant (B.1.617.2) has become more prevalent in Hong Kong and around the world.9 Apart from the high infectivity, what makes the Delta variant so prominent is the existence of gene mutations on the receptor-binding domain which are the important sites of the spike protein.10 A large number of mutations occurring on these sites would weaken the immune response triggered after infection, and also reduce the antibody neutralization induced by vaccines.10 Hence, more discussions should be focused on the necessity of an additional dose for the prevention of COVID-19 infection associated with variants. Dr. Lam proposed several points to be considered before the administration of a booster dose, including hosts’ immune status and risk of exposure, the durability of vaccines (more than 6 months), effectiveness towards Delta variant, and supplies of vaccines. Dr. Lam concluded, “Although there are uncertainties including the need for extra doses, vaccination is still the most important intervention against COVID-19 when considering the 6-month data of BNT162b2 and the efficacy towards severe diseases.”

