MEETING HIGHLIGHT
Redefining AD management: Lecanemab introduces new formulation backed by 4 years of positive efficacy and safety data
Alzheimer’s disease (AD) is now recognized as a progressive condition that begins silently, with harmful changes occurring years before symptoms appear.1-3 Timely intervention at its early stages is critical to slowing its progression.4 Lecanemab, a monoclonal antibody with a dual-targeting mechanism, not only targets plaques but also clears toxic amyloid-beta (Aβ) protofibrils—early drivers of neurodegeneration.5-8* At the 2025 Alzheimer’s Association International Conference (AAIC), new findings from the 48-month Clarity AD openlabel extension (OLE) study were unveiled along with real-world evidence and subgroup analyses, collectively underscoring lecanemab’s potential to slow cognitive decline and support functional independence across diverse patient populations.9,10 These findings reinforce its role as a disease-modifying therapy with long-term benefits.9,10 Data on subcutaneous (SC) lecanemab were also presented, offering insights into how an alternative formulation may enhance patient convenience whilst maintaining its efficacy.11
The continuous progression of early AD pathology*
The understanding of AD has evolved from a condition of late-life dementia to a "continuous" disease.1-3 AD can begin silently years before symptoms appear, with an insidious constellation of pathological changes, including the silent accumulation of the most neurotoxic species such as Aβ oligomers and protofibrils, that drives disease progression over time.2,3 Studies have shown that the process unfolds in a clear sequence: First, initial Aβ pathology emerges, these protein aggregates and plaques then spread throughout the brain, ultimately leading to a rise in tau pathology and other biological markers of neurodegeneration.2 Lecanemab is a monoclonal antibody designed to preferentially bind and clear highly toxic protofibrils.4,5 By neutralizing the most pathogenic form of Aβ at a critical early stage, the goal is to prevent the initiation of the downstream neurotoxic cascade before substantial and irreversible neuronal damage occurs.4,5 This foundational understanding of AD pathology sets the stage for evaluating long-term clinical outcomes with disease-modifying therapies like lecanemab.
*In-vitro data involved
Long-term efficacy of lecanemab from Clarity AD 48-month OLE
Building on its mechanistic rationale, lecanemab's long-term efficacy was described in the phase 3 Clarity AD study and its 48-month OLE.9 The pivotal Clarity AD study and its subsequent OLE have provided long-term evidence of lecanemab's efficacy.7,9 In the 18-month core study, lecanemab demonstrated a significantly slower decline on the Clinical Dementia Rating-Sum of Boxes (CDR-SB).7 The CDR-SB is scored from 0 to 18, evaluating areas like memory, orientation, judgment, problem-solving, daily activities, and personal care, where a higher score reflects more severe impairment.7 Patients receiving lecanemab scored lower than the placebo arm (1.21 vs. 1.66), resulting in a 27% difference (-0.45; 95% CI: -0.67 to -0.23; p<0.001).7,12
At the 48-month OLE, lecanemab-treated patients continued to accrue benefits over time, with more than 50% of patients remaining on lecanemab treatment.9† The OLE contrasted the Clarity AD cohort at 48 months with the Alzheimer's Disease Neuroimaging Initiative (ADNI) study, and the BioFINDER study, which are representative of the control AD population.9 The ADNI cohort was matched with the Clarity AD cohort by age, APOE ε4 carrier status, disease staging, and baseline CDR-SB.9 As for the BioFINDER cohort, patients only had mild cognitive impairment (MCI), showing better baseline cognitive reserve.9 The lecanemab group showed a progressively slower decline on the CDR-SB score over four years, effectively increasing the time patients spent in the earlier stages of disease (figure 1).9 At 18 months, the group showed a 0.52-point smaller decline compared with the ADNI cohort, representing a 5.0-month delay in progression.9 This gap widened to a 1.75-point difference by four years, which equates to a 10.7-month delay.9 Compared with the BioFINDER cohort, the benefit was even more pronounced, with a 0.57-point difference at 18 months (a 5.5-month delay) that grew to a 2.17-point difference at four years (a 13.1-month delay).9 Notably, at 48 months, 81.4% of patients on lecanemab retained in MCI or mild AD dementia stage with no progression.9
†At 48 months of the Clarity AD OLE study, 55.6% of patients remained on lecanemab treatment.9 This figure is based on the 859 patients in the lecanemab arm, of whom 478 continued treatment at 48 months.9 The percentage was calculated as follows: 478/859x100%=55.6%.
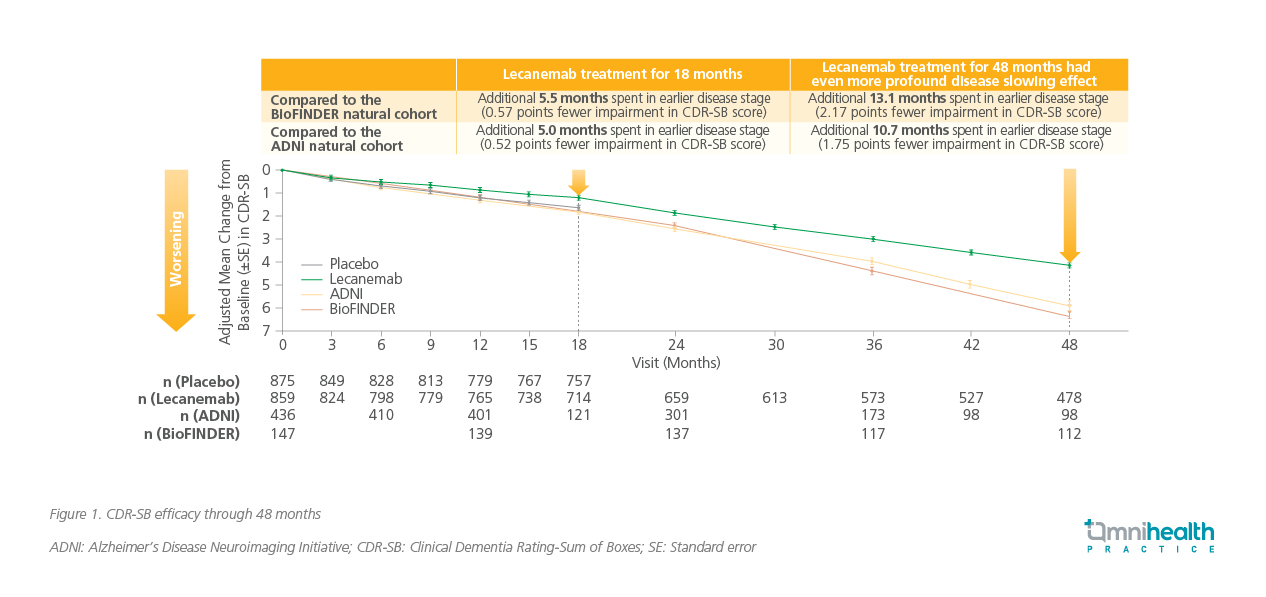
Compared to ADNI, lecanemab reduced the relative risk of progression to the dementia stage of the disease by 56% (HR=0.439; 95% CI: 0.336-0.573; p<0.00001).9 Data from the clinical trial is translated to real-life populations with interim data from a retrospective real-world study conducted in the United States (US) showing that after an average of about one year on lecanemab, 83.6% of AD patients on lecanemab either remained stable or clinically improved.10 The proportion of stable patients was 76.9% at the time of chart extraction, and was maintained at 80.9% at 6 months.10 Among those who stayed on treatment for 18 months, the proportion of patients who improved from mild AD dementia to MCI due to AD was 20%.10 These real-world findings reinforce the clinical trial results, highlighting lecanemab's potential to deliver meaningful benefits across broader patient populations.
Safety across the spectrum: Lecanemab by genetic risk and anticoagulant use
With efficacy established, safety across diverse populations becomes a key consideration in evaluating lecanemab's role in long-term treatment strategies. Both clinical trial and real-world data show that lecanemab is generally well tolerated, with amyloid-related imaging abnormalities (ARIA) events typically occurring within the first 6 months, stabilizing through 48 months, and rarely leading to serious outcomes.9,10 ARIA are particularly important in the monitoring of anti-amyloid monoclonal antibody therapies.10 In the US real-world study, the rates of ARIA were actually lower than those of Clarity AD trial (table 1), and the overall risk of ARIA remained low.10 To support safe use, magnetic resonance imaging (MRI) monitoring is recommended before the 5th, 7th, and 14th infusions.6 It is important to note that the following safety data were from lecanemab used as the sole anti-amyloid therapy.
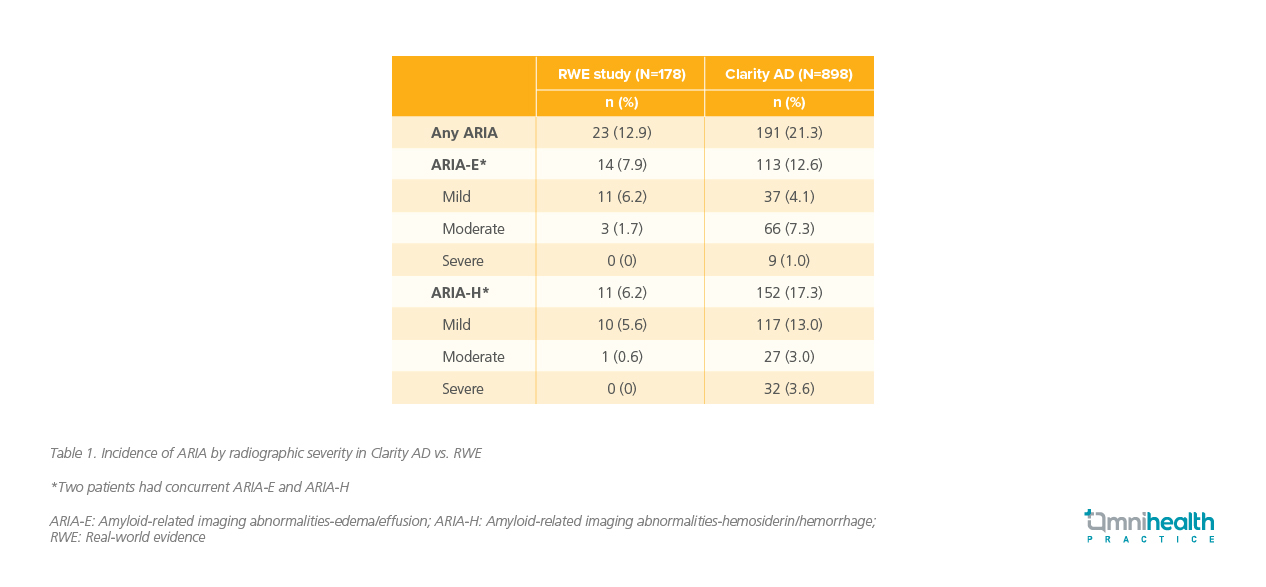
APOE ε4 is a known risk factor for ARIA, particularly in homozygous carriers.10 The pivotal Clarity AD trial included a significant proportion of APOE ε4 carriers (68.6%).9 The 48-month OLE demonstrated a favorable safety profile.9 Notably, initial analysis showed that ARIA rates were comparable between APOE ε4 carriers and non-carriers, despite the carriers' heightened genetic risk.7 In the US real-world study, 18.1% of patients were APOE ε4 homozygous, comparable to the roughly 15% prevalence in the general population.10 In the real-world study, the incidence of ARIA among APOE ε4 carriers—9.8% in heterozygous and 20.0% in homozygous individuals—was notably lower than the rates observed in the Clarity AD trial, which reported 19.0% and 45.0% respectively.10
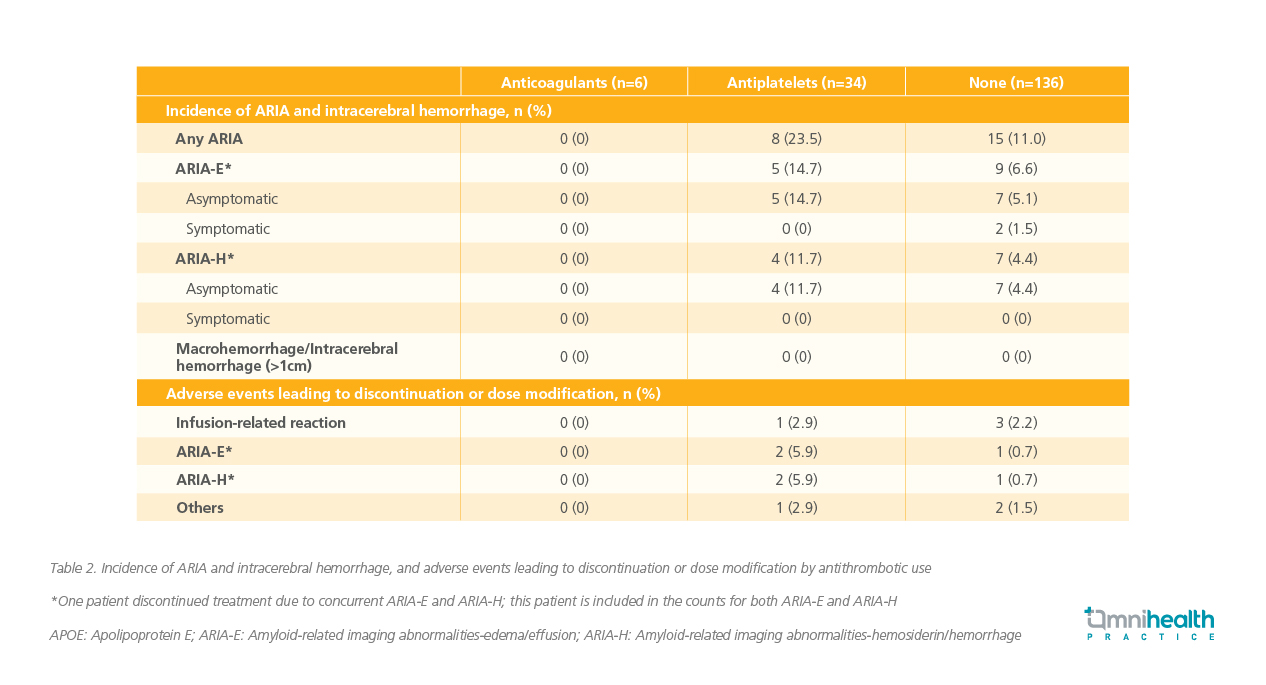
Aligning with data from Clarity AD where the risk stabilized after six months, most cases of ARIA in the US real-world study (77.3%‡) occurred within the first four months of treatment.10 The majority of ARIA events were also asymptomatic, and no macrohemorrhages, intracerebral hemorrhages, or deaths were reported (table 2).10 Additionally, among patients receiving concurrent anticoagulant therapy, the risk of ARIA remained low.10 In the real-world study, more frequent MRI testing was conducted in patients receiving anticoagulant therapy, and all doctors treated patients who were on antiplatelet medicine.10 As lecanemab moves into broader use, optimizing how it is administered will be crucial for long-term success and patient satisfaction.
‡The calculation of 77.3% was derived from the US real-world study.10 In this study, 17 ARIA events occurred at <4 months, while 5 ARIA events occurred at ≥4 months (range: 4-20 months).10 The percentage was calculated as follows: 17/(17+5)x100%=77.3%
Enhancing continued benefit with long-term SC autoinjector management
In a separate OLE study, patients had a treatment-free period of approximately 24 months before being reinitiated on lecanemab.8 Despite maintaining its clinical effectiveness relative to placebo, biomarker data show that after stopping lecanemab, plasma Aβ42/40 ratio and p-tau181 levels quickly revert toward pre-treatment levels, indicating re-accumulation of amyloid pathology.8 Lecanemab rapidly reduces amyloid levels within 3 months and shows clinical efficacy by 6 months, with over 80% of patients becoming amyloid-negative by 12-18 months, and a low incidence of ARIA-E.13 Continued dosing is necessary to sustain amyloid reduction, as long-term treatment helps maintain biomarker improvements and potentially slows disease progression.8 Data suggest potential disease-modifying effects and support the use of plasma biomarkers for monitoring and adjusting treatment, though further evaluation is ongoing.13
To facilitate long-term adherence and easier administration, the FDA has approved an SC formulation of lecanemab in early 2025.14 SC therapy offers a flexible initiation timeline, allowing patients to begin treatment anytime after completion of the core 18 months of IV regimen.4,14 SC weekly maintenance dosing provides bioequivalent drug exposure compared to the approved biweekly IV dose.4 Transitioning to the weekly 360mg SC lecanemab autoinjector after 18 months of IV dosing (10mg/kg every two weeks) maintains comparable clinical and biomarker benefits in early AD.14 Among 49 patients who made this switch, none experienced injection-related adverse events, and across over 600 patients studied, SC dosing showed a significantly lower rate of systemic reactions (<1%) compared to IV infusions (~26%), with ~11% reporting mild-to-moderate local reactions that did not interfere with treatment.14 ARIA rates were consistent with IV treatment and background levels, mostly occurring within the first 6 months of IV initiation.14 Additionally, a study evaluating the acceptability of the lecanemab autoinjector among patients, care partners, and healthcare professionals (n=126) found that it was well-received by users.11 Most found it easy to administer without assistance, were very confident using it (even at home), and reported high satisfaction and convenience.11 The device was well-received, with 100% of participants welcoming its introduction for medication administration.11 Overall, SC therapy offers a more convenient alternative to IV administration, with promising efficacy and patient-centered benefits.
Conclusion
Lecanemab represents a significant advancement in the treatment of AD, offering both clinical efficacy and a favorable safety profile across diverse populations. The Clarity AD trial and its 48-month extension provide compelling evidence of slowed disease progression and preserved independence, while real-world data reinforce its impact beyond controlled settings. Importantly, the introduction of the SC autoinjector marks a pivotal shift toward patient-centered care, reducing systemic reactions and improving treatment accessibility. As AD continues to challenge healthcare systems globally, lecanemab’s dual promise of early intervention and sustained benefit positions it as a cornerstone in the evolving standard of care. With ongoing research and broader adoption, the future of Alzheimer’s treatment is becoming clearer—and more hopeful.

