FEATURES
Neurodegeneration and PSE risk factors in addition to new development of personalized treatments
In the 2022 Post-14th European Epilepsy Congress (EEC) Symposium, Dr. Hui, Ting-Hin Adrian shared his insights into neurodegeneration epilepsy and post-stroke epilepsy (PSE). He highlighted the risk factors for both types of epilepsy and suggested the adoption of proper treatments, while acknowledging the current limitations in this field. Following his presentation, Dr. Richard Chang dived deep into a topic relating to personalized medicine, which enables patients with epilepsy to receive proper and more effective treatment. He continued by sharing his group’s study on brivaracetam, demonstrating its efficacy and tolerability in seizure control among the Chinese population.
The relationship between neurodegeneration and epilepsy
There exists a bidirectional and u-shaped relationship between dementia and epilepsy, which means that while dementia could be a cause of epilepsy, in return, epilepsy may also be a risk factor in the development of dementia.1 For example, as illustrated by Dr. Hui, patients suffering from fluctuating consciousness caused by dementia are at a higher risk of falls, which could cause post-traumatic epilepsy (PTE) or subdural hematoma (SDH). Such conditions will lead to cognitive decline, which increases the risk of seizures. The inverse is also true: Seizures are associated with cognitive decline among patients. This could increase the risk of falls and fluctuating states of consciousness. Statistics have shown that the incidence of epilepsy increased with age, justifying that the elderly population is, therefore, at a higher risk of neurodegeneration-induced epilepsy.1
Combination of AD and hyperexcitability causes epilepsy
Alzheimer’s disease (AD) is the third most common etiology of epilepsy in the elderly, behind stroke and neoplasm.1 It is reported that patients with AD experience more unprovoked seizures, and that patients with AD with subclinical epileptiform activity develop cognitive decline 5.5 years earlier than patients without epilepsy.2 The association with AD and hyperexcitability is well established. Firstly, gene-related factors are notable. The apolipoprotein E4 (APOE4) gene and amyloid precursor protein (APP) gene or presenilin mutation increase subclinical epileptiform activity.3 A well known example is trisomy 21 (i.e., down syndrome), which increases the expression of APP, leading to epilepsy.4 In addition, inhibitory entorhinal cortical interneurons in AD patients are more susceptible to oxidative stress, leading to earlier cell death.5 This leads to the disinhibition of excitatory neurons-causing hyperexcitability.5 Lastly, the accumulation of phosphorylated-tau (p-tau) and beta-amyloid can increase calcium-mediated signals, again resulting in neurotoxicity and excitability.5 Owing to these factors, cognitive decline is established and closely linked with epilepsy.
Preferred ASMs for the elderly
Dr. Hui recommended that anti-seizure medications (ASMs) should be started with the first episode of unprovoked seizure in an elderly patient with cognitive impairment. An ideal ASM should have the following features: Easy to administer with good efficacy; minimal toxicity, particularly relating to cognitive impairment, and minimal to no drug-drug interactions (DDIs). However, he cautioned that even if an ideal medication is available, intrinsic factors would increase the risk of toxicity. Such factors include age-related reduction in the hepatic synthesis rate, hepatic metabolic rate, and renal elimination rate.6 This stresses the importance of choosing a proper medication by taking into consideration each patient's medical condition. For example, in patients with liver dysfunction, valproic acid should be avoided, whereas levetiracetam would be a suitable option.7 Other important factors for choosing ASMs include cognitive impairments, osteoporosis, psychiatric illnesses, tremors, and cerebellar degeneration.8-12 Certain drugs may have concomitant psychotropic properties, such as being an antidepressant, mood-stabilizing, or anxiolytic.13 Therefore, mental states of patients should also be taken into account. Dr. Hui emphasized that it is unnecessary to withdraw ASM even if this group of patients has remained seizure-free for years. “For patients who are demented and having a seizure, it is better not to withdraw ASM because the neurodegeneration is irreversible, and yet it will get worse along with seizures,” he explained.
Early vs. late PSSs
Post-stroke seizure (PSS) is also common. Early and late PSSs are generally distinguished using the 7-day rule, which defines seizures within 7 days of stroke onset to be an early PSS, and seizures occurring after 7 days to be late PSS.14 These 2 types of PSS have different prognoses. Early PSS, labeled as symptomatic, occurs in 3%-6% of stroke patients and has a lower risk of seizure recurrence over the next 10 years at 33%.15,16 Late PSS, labeled as unprovoked, occurs in 10%-12% of stroke patients and has a high risk of seizure recurrence (71.5%) over the next 10 years.15 It is also shown that early and late PSSs differ in pathophysiology.15 Early PSS is caused by transient cellular biochemical dysfunctions with the release of glutamate and free fatty acids.15 It is characterized by homeostatic or systemic disturbances, such as electrolyte imbalance, acid-base disturbances, and hyperglycemia.15 On the other hand, late seizures involve structural changes, such as hemosiderin deposits and epileptogenic gliotic scarring or apoptosis, and are associated with selective neuronal loss and mitochondrial changes.15 Consequently, while early and late PSSs share certain risk factors, such as cortical involvement, intracerebral hemorrhage (ICH) and stroke severity, each of them also has its own risk elements.17 For example, alcohol consumption will increase the risk of early PSS, while early PSSs and younger age at onset are the risk factors for late PSS.17,18
Recommended treatment approaches for early and late PSSs
Dr. Hui then presented the recommended treatments for early and late PSSs. He explained that the thrombolytic, recombinant tissue plasminogen activator could be administered during acute stroke without increasing the incidence of PSS.19 However, secondary prophylaxis is generally not indicated for early PSS, unless there are multiple recurrent seizures within 24 hours, a risk of clinical worsening, or conditions that increase the risk of seizure recurrence such as venous thrombosis, intracranial hemorrhage or subarachnoid hemorrhage.16 Even so, Dr. Hui recommended that under these situations, secondary prophylaxis should be stopped after the acute phase. Some specific conditions, however, do require treatment beyond the acute phase. This includes post-stroke status epilepticus (PSSE) due to its high, risk of seizure recurrence (i.e., 41% in 10 years); patients with the National Institutes of Health Stroke Scale (NIHSS) score >4; and, PSSE duration >16 hours.20 Dr. Hui also noted that the definition of “acute phase” varies, saying that “some may suggest to be around 1 week, 1 month, or 3 months. It really depends on the clinical setting.” As for treating late PSS, Dr. Hui noted that current research on this aspect is limited. There were 2 randomized, open-label trials.21,22 One compared lamotrigine and controlled-release carbamazepine (CBZ-CR), while the other compared levetiracetam and CBZ-CR.21,22 Both trials showed no difference in efficacy, but lamotrigine and levetiracetam were better tolerated.21,22 Research on primary prophylaxis (i.e., antiepileptoenesis) after stroke is still ongoing.23
The concepts and benefits of personalized precision medicine
Dr. Richard Chang then introduced the concepts and benefits of personalized precision medicine, which aims to provide the right treatments to the right patients at the right time, instead of using a one-size-fits-all strategy.24 It can be divided into several levels, with higher levels corresponding to higher specificity of the treatment (figure 1). For example, the lower levels involve recognized responses to certain ASMs in specific epilepsy types, perhaps in genetic epilepsies.25 Whereas the upper levels involve therapies directly targeting protein dysfunction, messenger ribonucleic acid (mRNA), or gene defects.25
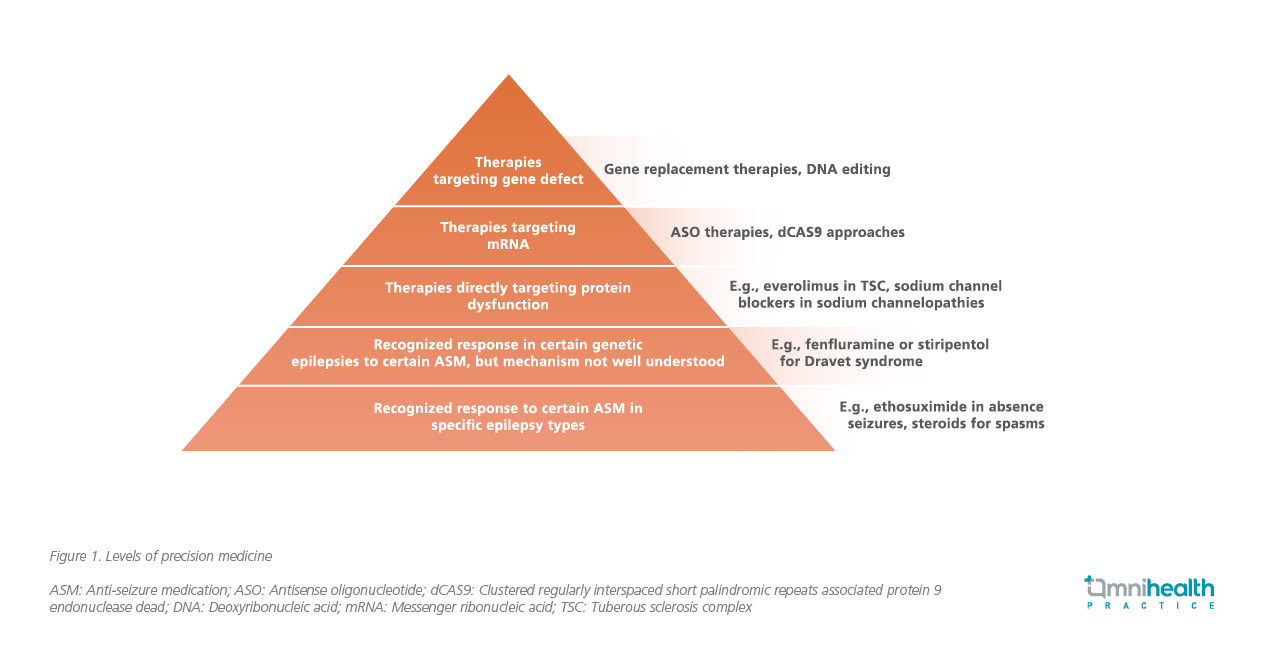
Techniques such as blood tests, gene sequencing, and imaging could be used to identify biomarkers that guide the treatment.25,26 Dr. Chang also mentioned the benefits of personalized medicine in therapeutic drug monitoring (TDM). While TDM is useful for identifying changes in kinetics and assessing exposure, systematic TDM is limited in its utility.27,28 As an example, seizure freedom is usually reached below the reference ranges, and that some patients may be able to tolerate and require doses above the upper limit of the reference range.28 It is suggested that taking a personalized approach of considering an “individual therapeutic concentration/range” could be more useful compared with using the population reference range.28 In addition, personalized medicine may also be employed to help predict adverse events (AEs).24 By identifying different alleles in genetic makeup and considering the associated AEs, physicians could evaluate the risks of different AEs manifesting.24 This could further be employed to bring certain “lost ASMs” back into usage.24 “Lost ASMs” refer to ASMs that are rarely prescribed due to their possible AEs, such as vigabatrin, which could cause vision defects or even vision loss.29 Personalized medicine allows the risk of each patient to be identified.24 If the patient has a low risk of experiencing such AEs, then the “lost ASMs” could be viable treatment options.24 Though research is still ongoing in large parts of this field, as explained by Dr. Chang, new developments such as using machine learning to predict drug response might bring substantial benefits to patients in the future.30
Brivaracetam demonstrates superior efficacy and tolerability in seizure control
Dr. Chang presented his group’s findings on the efficacy and tolerability of brivaracetam in the Chinese population.31 Patients with epilepsy who were prescribed brivaracetam were eligible for enrollment.31 The study compared the effects of brivaracetam with levetiracetam by assessing seizure frequencies within 12 weeks before and after the initiation of brivaracetam.31 The study also determined the number of patients experiencing good response to brivaracetam, defined as either maintenance of seizure freedom or ≥50% reduction in seizure frequency after brivaracetam.31 The number of patients who were maintained on brivaracetam 12 weeks after initiation was also evaluated.31 Lastly, treatment-emergent adverse events (TEAEs) were also studied to evaluate the safety profile of brivaracetam.31
A total of 66 patients with a 1:1 male-to-female ratio were enrolled.31 At baseline, 76% of patients had drug-resistant epilepsy (DRE) and 26% had psychiatric comorbidities.31 About 76% of patients switched from levetiracetam.31 The mean and median seizure frequencies in the 12 weeks before initiation were 3.8 and 2.0 per month, respectively.31 In the 12 weeks after initiation, the mean and median frequencies were similar, at 4 and 2 per month, respectively.31 In total, 27% of patients were deemed to be good responders and 20% remained seizure-free, with 5% newly achieving seizure freedom.31 About 11% of patients experienced TEAEs, and the common TEAEs included malaise, drowsiness, and mood disturbances.31 A negative correlation between DRE and good response to brivaracetam was found [odds ratio (OR)=82.05; 95% CI: 10.56-637.87; p<0.01] and 86% of patients were still taking brivaracetam on extended follow-up at week 36, showing good retention.31 The study demonstrated that brivaracetam was effective in providing seizure control in the Chinese population and the results were comparable to local data of lacosamide and perampanel when compared with previous landmark studies.31,32 The high retention rate (i.e., 88% at week 12) also suggested good tolerability and safety of brivaracetam among patients.31
Conclusion
To sum up, epilepsy and dementia have a bidirectional relationship, particularly with AD.1 Though some medications are available for this group of patients, great care must be taken when considering the possible side effects.8-13 The use of personalized medicine, which aims to adapt treatment to each individual, could help this group of patients by identifying the proper treatment and predicting the possible AEs.24 In addition to neurodegeneration-induced epilepsy, stroke also presents complications to the development of epilepsy, with PSE being divided into early and late stage.14 Of note, a new study has shown that brivaracetam was effective and safe in providing seizure control among the Chinese population, enhancing our knowledge towards the treatment of seizures.31
NAME OF THE MEDICINE PRODUCT - BRIVIACT (brivaracetam)
Active Ingredient: Brivaracetam - Tablets: 25, 50 and 100mg. Oral Solution: 10mg per ml. Injection/Infusion: 10mg per ml. Indications: Adjunctive therapy in the treatment of partial-onset seizures with or without secondary generalisation in adult and adolescent patients from 16 years of age with epilepsy. Dosage and Administration: Starting dose of 50 or 100mg/day, adjusting up to 200mg/day. All daily doses to be given in two equally divided doses, morning and evening with or without food. All dose adjustments based on physician’s assessment, patient response and tolerance. Swallow tablets whole with liquid. Oral solution may be diluted in water or juice shortly before administration orally, via nasogastric or gastrostomy tube. Injection/ infusion may be given as an intravenous bolus or diluted in a compatible solution and administered as a 15-minute intravenous infusion. Injection/ infusion is not recommended in acute conditions, such as status epilepticus. Renal impairment: No dose adjustment is needed but not recommended in end-stage renal disease patients undergoing dialysis due to lack of data. Hepatic impairment: Exposure is increased in chronic liver disease - see full prescribing information for dose guidance. Elderly: Limited experience but no dose adjustment needed. Discontinuation: Withdraw gradually – see full prescribing information for dose guidance. Contraindications: Hypersensitivity to brivaracetam, other pyrrolidone derivatives or to any of the excipients listed in the full prescribing information. Warnings: Suicidal ideation and behaviour have been reported in patients treated with anti-epileptic drugs (AEDs). Patients should be monitored and appropriate treatment considered. Dose adjustment recommended in hepatic impairment. Tablets not to be taken by patients with galactose intolerance, total lactase deficiency or glucose-galactose malabsorption. Interactions: Brivaracetam plasma concentrations may increase with CYP2C19 strong inhibitors (risk of a clinically relevant CYP2C19 mediated interaction considered to be low). Brivaracetam plasma concentrations decreased with strong enzyme inducing AEDs but no dose adjustment is required. Caution and consider adjusting the brivaracetam dose in patients starting or ending treatment with rifampicin or St John’s Wort, strong inducers of CYP2C19. Brivaracetam may increase plasma concentrations of medicinal products metabolised by CYP2C19 or transported by OAT3 and decrease plasma concentrations of medicinal products metabolised by CYP2B6. Potential interactions between brivaracetam and other AEDs were investigated, please refer to the full prescribing information section 4.5 for full details. Concomitant alcohol intake is not recommended. Pregnancy and lactation: Discuss family planning and contraception with women of childbearing potential taking brivaracetam. As a precaution, should not be used during pregnancy unless clinically necessary. It is unknown whether brivaracetam is excreted in human breast milk so a clinical decision needs to be made as to whether to discontinue either breastfeeding or brivaracetam. Driving and operate machinery: Brivaracetam has minor or moderate influence on the ability to drive and use machines (may include somnolence, dizziness, and other central nervous system related symptoms). Patients should be advised not to drive or to operate other potentially hazardous machines until familiar with the effects of brivaracetam. Adverse Effects: Very Common (≥1/10): Dizziness, somnolence. Common (≥1/100 - <1/10): Influenza, decreased appetite, depression, anxiety, insomnia, irritability, convulsion, vertigo, upper respiratory tract infections, cough, nausea, vomiting, constipation, fatigue. Uncommon (≥1/1000 - <1/100) neutropenia, type 1 hypersensitivity, suicidal ideation, psychotic disorder, aggression, agitation. See full prescribing information for further details. Pharmaceutical Precautions: Oral solution: Use within 5 months of opening. Injection/Infusion: Use immediately after dilution.
Please refer to the full prescribing information before prescribing.
Further information is available from: UCB Pharma (Hong Kong) Limited
Ref.: HK PI 13-Sep-202
NAME OF THE MEDICINAL PRODUCT - VIMPAT (lacosamide)
PHARMACEUTICAL FORM Film-coated tablets containing 50mg or 100mg lacosamide. Solution for infusion containing 10 mg lacosamide per ml. Syrup containing 10mg lacosamide per ml. THERAPEUTIC INDICATIONS Vimpat is indicated as monotherapy and adjunctive therapy in the treatment of partial-onset seizures with or without secondary generalisation in adults, adolescents and children from 4 years of age with epilepsy. Vimpat is indicated as adjunctive therapy • in the treatment of partial-onset seizures with or without secondary generalisation in adults, adolescents and children from 4 years of age with epilepsy. • in the treatment of primary generalised tonic-clonic seizures in adults, adolescents and children from 4 years of age with idiopathic generalised epilepsy. POSOLOGY AND METHOD OF ADMINISTRATION Lacosamide must be taken twice a day (usually once in the morning and once in the evening). Tablets/Syrup: Lacosamide may be taken with or without food. IV: Lacosamide therapy can be initiated with either oral or i.v. administration. The overall duration of treatment with i.v. lacosamide is at the physician’s discretion; there is experience from clinical trials with twice daily infusions of lacosamide for up to 5 days in adjunctive therapy. Conversion to or from oral and intravenous administration can be done directly without titration. Monitor closely patients with known cardiac conduction problems, on concomitant medications that prolong PR interval, or with severe cardiac disease (e.g. myocardial ischemia, heart failure) when lacosamide dose is higher than 400 mg/day. Adults, adolescents and children weighing 50kg or more Starting dose (monotherapy for partial onset seizures and adjunctive therapy for partial onset seizures or primary generalised tonic-clonic seizures) - 50mg twice a day. Increase to initial therapeutic dose of 100mg twice a day after 1 week. For monotherapy, dose can also be initiated at 100mg twice a day. Maintenance dose (monotherapy for partial onset seizures and adjunctive therapy for partial onset seizures or primary generalised tonic-clonic seizures) – dose can be further increased at weekly intervals by 50mg twice a day, up to a max recommended daily dose of 300mg twice a day for monotherapy or 200mg twice a day for adjunctive therapy. Loading dose (initial monotherapy or conversion to monotherapy in the treatment of partial onset seizures or adjunctive therapy in the treatment of partial-onset seizures or adjunctive therapy in the treatment of PGTCS) - May also initiate with a single loading dose of 200 mg, followed approximately 12 hours later by a 100 mg twice a day (200 mg/day) maintenance dose regimen. Administered under medical supervision with consideration of the potential for increased incidence of serious cardiac arrhythmia and central nervous system adverse reactions. Administration of a loading dose has not been studied in acute conditions such as status epilepticus. Discontinuation - If lacosamide has to be discontinued, recommended to do it gradually (e.g. taper the daily dose by 200 mg/week). If patient develops serious cardiac arrhythmia, perform clinical benefit/risk assessment and discontinue lacosamide if needed. Elderly (over 65 years of age) - No dose reduction is necessary in elderly patients. Renal impairment - No dose adjustment is necessary in mildly and moderately renally impaired adult and paediatric patients (CLCR >30 ml/min). In paediatric patients weighing 50kg or more and in adult patients with mild or moderate renal impairment, a loading dose of 200 mg may be considered, but further dose titration (>200 mg daily) should be performed with caution. In paediatric patients weighing 50kg or more and in adult patients with severe renal impairment (CLCR ≤30 ml/min) or with end-stage renal disease, a maximum dose of 250 mg/day is recommended and the dose titration should be performed with caution. Hepatic impairment - A maximum dose of 300mg/day is recommended for paediatric patients weighing 50kg or more and for adult patients with mild to moderate hepatic impairment. Paediatric population - The physician should prescribe the most appropriate formulation and strength according to weight and dose. The dose for paediatric patients (from 4 years of age) and adolescents weighing less than 50kg is determined based on body weight. Please refer to the package insert for dosage recommendation in this population. CONTRAINDICATIONS Hypersensitivity to the active substance or to any of the excipients. Known second- or third-degree atrioventricular (AV) block. SPECIAL WARNING AND PRECAUTIONS FOR USE Suicidal ideation and behaviour have been reported in patients treated with anti-epileptic medicinal products in several indications. Patients should be monitored for signs of suicidal ideation and behaviours and appropriate treatment should be considered. Patients (and caregivers of patients) should be advised to seek medical advice should signs of suicidal ideation or behaviour emerge. Dose-related prolongations in PR interval with lacosamide have been observed in clinical studies. Lacosamide should be used with caution in patients with underlying proarrhythmic conditions such as patients with known cardiac conduction problems or severe cardiac disease or patients treated with medicinal products affecting cardiac conduction, including antiarrhythmics and sodium channel blocking antiepileptic medicinal products as well as in elderly patients. Consider performing ECG before dose increase to above 400mg/day and after lacosamide is titrated to steady state. Patients should be made aware of the symptoms of cardiac arrhythmia. Patients should be counselled to seek immediate medical advice if these symptoms occur. New onset or worsening of myoclonic seizures has been reported in both adult and paediatric patients with PGTCS, in particular during titration. Patients should be advised to exercise caution until they are familiar with the potential effects of the medicine to prevent accidental injury or falls. The safety and efficacy of lacosamide in paediatric patients with epilepsy syndromes in which focal and generalised seizures may coexist have not been determined. Vimpat solution for infusion contains sodium. To be taken into consideration for patients on a controlled sodium diet. INTERACTION WITH OTHER MEDICINAL PRODUCTS AND OTHER FORMS OF INTERACTION Use with caution in patients treated with medicinal products known to be associated with PR prolongation (including sodium channel blocking antiepileptic medicinal products) and in patients treated with antiarrhythmics. Caution when use with strong inhibitors of CYP2C9 (e.g. fluconazole) and CYP3A4 (e.g. itraconazole, ketoconazole, ritonavir, clarithromycin), and strong enzyme inducers such as rifampicin or St John’s wort (Hypericum perforatum). PREGNANCY AND BREASTFEEDING Lacosamide should not be used during pregnancy unless clearly necessary. Breast-feeding should be discontinued during treatment with lacosamide. UNDESIRABLE EFFECTS Very common (≥1/10): dizziness, headache, nausea and diplopia (usually mild to moderate in intensity). Common (≥1/100 to <1/10): depression, confusional state, insomnia, myoclonic seizures, ataxia, balance disorder, memory impairment, cognitive disorder, somnolence, tremor, nystagmus, hypoesthesia, dysarthria, disturbance in attention, paresthesia, vision blurred, vertigo, tinnitus, vomiting, constipation, flatulence, dyspepsia, dry mouth, diarrhea, pruritus, rash, muscle spasms, gait disturbance, asthenia, fatigue, irritability, feeling drunk, fall, skin laceration, contusion. The use of lacosamide is associated with dose-related increase in the PR interval. Adverse reactions associated with PR interval prolongation (e.g. atrioventricular block, syncope, bradycardia) may occur. Paediatric Population - The safety profile in paediatric population was consistent with the safety profile observed in adults although the frequency of some adverse reactions (somnolence, vomiting and convulsion) was increased and additional adverse reactions (nasopharyngitis, pyrexia, pharyngitis, decreased appetite, lethargy and abnormal behaviour) have been reported in paediatric patients.
Please refer to the full prescribing information before prescribing.
Ref. FCT, SFI, OS – 24-Sep-2021

