CONFERENCE UPDATE
Extended reduced-dose apixaban as an effective and safer alternative to full-dose therapy in cancer-associated VTE: Results from the API-CAT study
STUDY DESIGN
International guidelines recommend maintaining anticoagulation for cancer-associated thrombosis for the duration of active malignancy or ongoing cancer therapy.1 As the risk of recurrent venous thromboembolism (VTE) decreases over time while bleeding risk remains substantial, reduced-dose apixaban was hypothesized to have non-inferior efficacy and improved safety compared to the full-dose therapy for extended VTE treatment.1
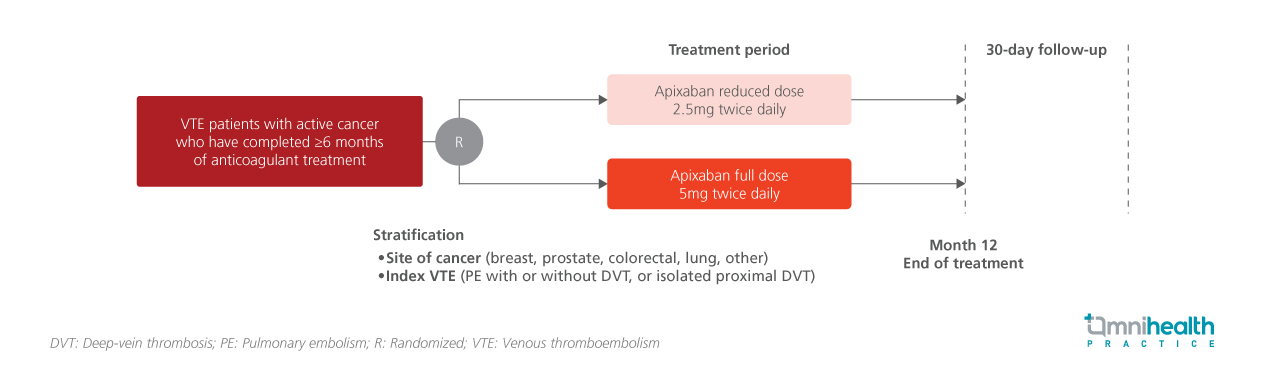
The API-CAT trial was a multicenter, randomized, double-blind study designed to evaluate the efficacy and safety of reduced-dose (2.5mg twice daily) vs. full-dose (5mg twice daily) apixaban for the extended treatment of VTE in patients with active cancer who had completed at least six months of anticoagulation therapy.1 The study aimed to demonstrate the non-inferiority of the reduced dose in preventing recurrent VTE and the superiority in reducing clinically relevant bleeding.1
A total of 1,766 patients were randomized 1:1 to receive either reduced-dose (n=866) or full-dose (n=900) apixaban.1 Eligible participants were patients with active cancer and a history of VTE (defined as proximal deep-vein thrombosis [DVT] of the lower limb or pulmonary embolism [PE]) who had completed at least six months of anticoagulant therapy.1 Among them, 24.5% had DVT only, while 75.5% had PE (with or without DVT).1 The median interval since the index event was 8.0 months.1 Breast cancer was the most common cancer type at 22.7%, and most of the participants (65.8%) had metastatic disease.1 The median duration of trial intervention was 11.8 months.1
The primary efficacy outcome was adjudicated fatal or nonfatal recurrent VTE, a composite of recurrent symptomatic VTE, incidental VTE, and VTE-related death, assessed in a noninferiority analysis.1 The key secondary safety outcome was adjudicated clinically relevant bleeding, a composite of major bleeding and clinically relevant non-major bleeding, evaluated for superiority.1
FINDINGS
| Primary outcome: |
|
|
|
| Key secondary safety outcome: |
|
|
|
“Our results indicate that patients with active cancer who have completed at least 6 months of anticoagulant treatment may be eligible to receive a reduced dose of apixaban for extended treatment
Dr. Isabelle Mahé
Assistance Publique–Hopitaux de Paris (AP-HP),
Colombes, France

