CONFERENCE UPDATE
Ravulizumab shows sustained efficacy and safety in preventing relapses in AQP4+ NMOSD: Interim results from the CHAMPION-NMOSD trial
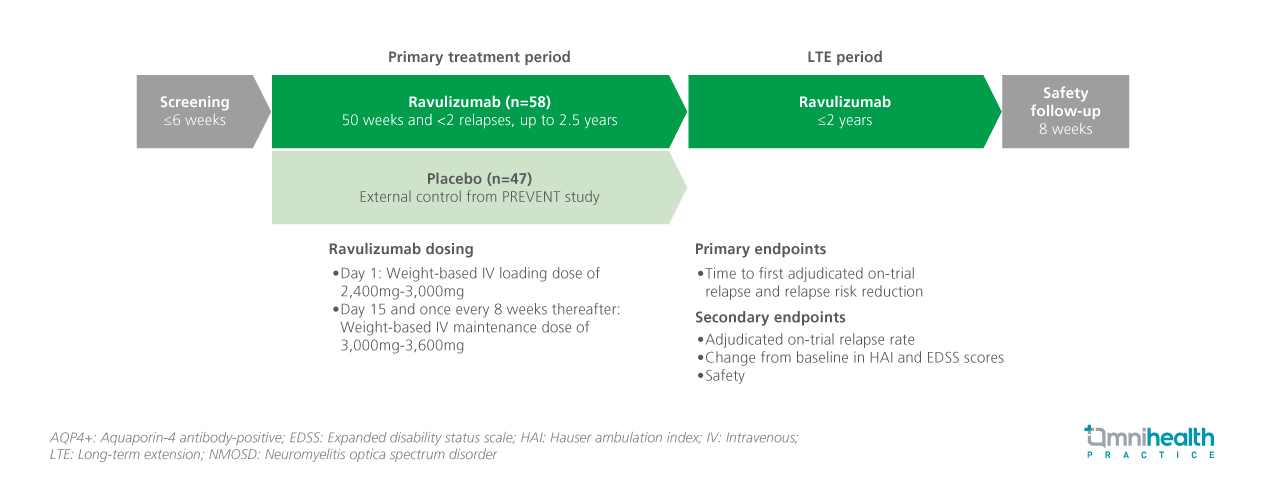
STUDY DESIGN
Aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder (AQP4+ NMOSD) is a rare autoimmune disease of the central nervous system characterized by recurrent and unpredictable relapses, which can lead to significant and irreversible neurological disability.1 Ravulizumab is a long-acting C5 complement inhibitor designed to prevent relapses in patients with AQP4+ NMOSD.1 This interim analysis presents long-term outcomes from the CHAMPION-NMOSD study, evaluating the efficacy and safety of ravulizumab in adults with AQP4+ NMOSD over a median follow-up period of 170.3 weeks.1
The CHAMPION-NMOSD trial is an ongoing, global, open-label, phase 3 study evaluating ravulizumab in patients with AQP4+ NMOSD.1 A total of 58 patients were enrolled in the primary treatment period (PTP), with 56 patients entering the long-term extension (LTE) phase as of the data cutoff on June 14, 2024.1 Adult participants in PTP received an intravenous weight-based loading dose of ravulizumab on day 1, followed by maintenance doses starting on day 15 and every 8 weeks thereafter.1 The placebo arm (n=47) of the PREVENT study, which evaluated eculizumab in patients with AQP4+ NMOSD, was used as an external control group.1 After completing the PTP for up to 2.5 years, patients were eligible to enter the LTE phase.1 Baseline demographics and clinical characteristics were generally similar between the ravulizumab group in CHAMPION-NMOSD and those in the placebo group from the PREVENT study.1
The primary endpoints of the study was the time to first adjudicated on-trial relapse and relapse risk reduction.1 Secondary endpoints included adjudicated relapse rate, and changes from baseline in Hauser Ambulation Index (HAI) and Expanded Disability Status Scale (EDSS) scores and safety profile.1
“These findings support the long-term clinical benefit of ravulizumab in preventing relapses and preserving neurological function in adults with AQP4+ NMOSD"
Dr. Sean J. Pittock
Mayo Clinic,
Minnesota, United States
FINDINGS
| Primary endpoint: | |
|
|
|
|
| Secondary endpoints: | |
|
|
|
|
|
|
|
|
| Safety: | |
|
|
|
|
|
|
|

