CONFERENCE UPDATE: AAN 2024
Final results from CHAMPION MG OLE demonstrated the long-term efficacy and safety of ravulizumab in adults with AChRAb+ gMG
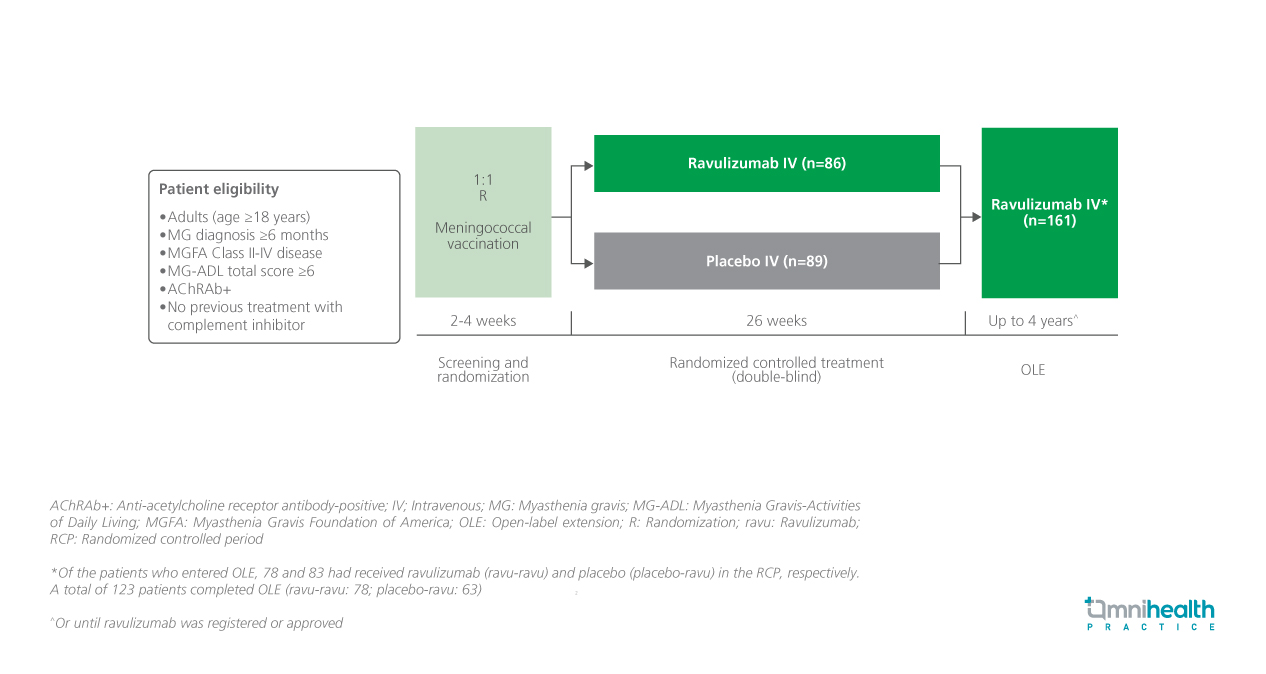
STUDY DESIGN
Autoantibodies against the acetylcholine receptor (AChR) are often found in patients with generalized myasthenia gravis (gMG), which destroy the post-synaptic membrane of the neuromuscular junction (NMJ) via aberrant complement cascade activation.1 Humanized monoclonal antibodies with a high affinity to the human terminal complement component C5 such as ravulizumab inhibit the membrane attack complex formation.1 Previously, the 26-week randomized controlled period (RCP) of the phase 3 CHAMPION MG study demonstrated the efficacy and safety profile of ravulizumab in adult patients with anti-acetylcholine receptor antibody-positive (AChRAb+) gMG.1
To evaluate the long-term efficacy and safety of ravulizumab in the indication, patients were also invited to enter the open-label extension (OLE) phase.1 Those who entered OLE (n=161) were given intravenous (IV) ravulizumab on a weight-based loading dose on day 1, proceeded to receive a weight-based maintenance dose on day 15, then every 8 weeks for up to 4 years.1 This analysis included data from these patients for up to 164 weeks (RCP: 26 weeks; OLE: 138 weeks).
Changes in patient-reported Myasthenia Gravis-Activities of Daily Living (MG-ADL) total score and physician-assessed Quantitative Myasthenia Gravis (QMG) total score from RCP and OLE baseline were assessed which were analyzed using a mixed-effect model for repeated measures (MMRM).1 Other endpoints included MG-ADL response rate, minimal symptom expression (MSE), corticosteroid (CS) use, as evaluated by changes in CS dose and proportion of CS treatment discontinuation.1 The safety and tolerability of ravulizumab were also assessed.1
FINDINGS
|
Efficacy endpoints: |
|
|
|
|
|
|
Safety: |
|
|
|
“Overall, these findings support the sustained clinical effectiveness and long-term safety of ravulizumab, administered every 8 weeks, in adults in AChRAb+ gMG”
Dr. Tuan Vu
University of South Florida Morsani College of Medicine,
Florida, United States

