CONFERENCE UPDATE: CROI 2025
Twice yearly lenacapavir demonstrates high efficacy for HIV prevention and safety in adolescents and adults: Insights from PURPOSE 1
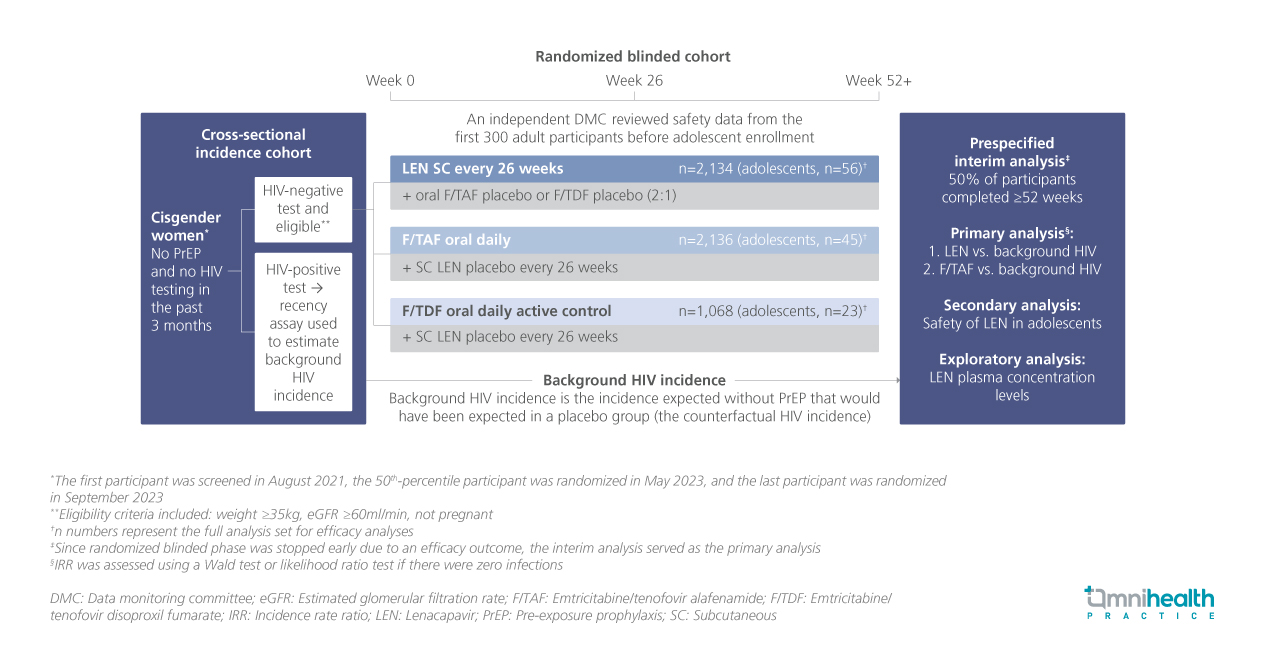
STUDY DESIGN
Despite the high incidence of human immunodeficiency virus (HIV) infection among adolescents, this population has historically been excluded from phase 3 HIV trials.1 As a result, clinical data on new therapeutic options in adolescents often become available years after adult approvals, delaying their access to pre-exposure prophylaxis (PrEP).1 Daily adherence to PrEP is also lower among adolescents than adults, highlighting the need for alternative strategies.1 Lenacapavir (LEN) is a first-in-class, multistage HIV-1 capsid inhibitor with high potency and a long half-life, supporting twice-yearly subcutaneous (SC) injections.1
The PURPOSE program is the first to intentionally include adolescents aged 16 and 17 years in an HIV prevention trial.1 PURPOSE 1 was a randomized, blinded trial designed to evaluate the efficacy, safety, and pharmacokinetics (PK) of LEN in adolescents compared to adults while implementing strategies to support adolescent participation.1
In PURPOSE 1, cisgender women at risk of HIV infection were assigned to receive LEN subcutaneously every 26 weeks (n=2,134; adolescents: n=56), daily oral emtricitabine + tenofovir alafenamide (F/TAF) (n=2,136; adolescents: n=45), or daily oral emtricitabine + tenofovir disoproxil fumarate (F/TDF) as the active control (n=1,068; adolescents: n=23).1 Before enrolling adolescents, an independent data monitoring committee reviewed safety data from the first 300 adult participants.1 The study also included a cross-sectional incidence cohort to estimate background HIV incidence.1 To ethically include adolescents, the study team engaged adolescent medicine experts and community advocates.1 Site-specific strategies ensured appropriate consent processes, including community advisory board-facilitated consent waivers and self-consent for emancipated minors.1 Trial sites also adapted procedures such as early morning and weekend visits to accommodate adolescent schedules.1 Prespecified analyses included primary comparisons of LEN and F/TAF against background HIV incidence, secondary assessment of LEN’s safety in adolescents, and exploratory evaluation of LEN plasma concentrations.1
FINDINGS
|
Efficacy endpoints: |
|
|
|
|
| Safety: |
|
|
|
|
|
| Exploratory analysis: |
|
|
"LEN demonstrated 100% efficacy for HIV prevention in adolescent and adult cisgender women"
Dr. Katherine Gill
Desmond Tutu HIV Centre,
University of Cape Town,
South Africa
Outcomes of NADIA trial at 96 weeks
After failure with emtricitabine (FTC) and 2 nucleoside reverse-transcriptase inhibitors (NRTIs), the recommended second-line treatment regimen for human immunodeficiency virus (HIV) from the World Health Organization (WHO) is dolutegravir (DTG) with 2 NRTIs, tenofovir disoproxil fumarate (TDF) or zidovudine (ZDV).1 The efficacy of this regimen has not been proven as the activity of NRTIs is thought to be limited due to drug resistance.2 Similarly, drug resistance is expected when switching TDF to ZDV.2

