CONFERENCE UPDATE: 2025 AAAAI/WAO Joint Congress
Dupilumab improves histologic, symptomatic, and endoscopic features of EoE regardless of concurrent FED: Post hoc LIBERTY analysis
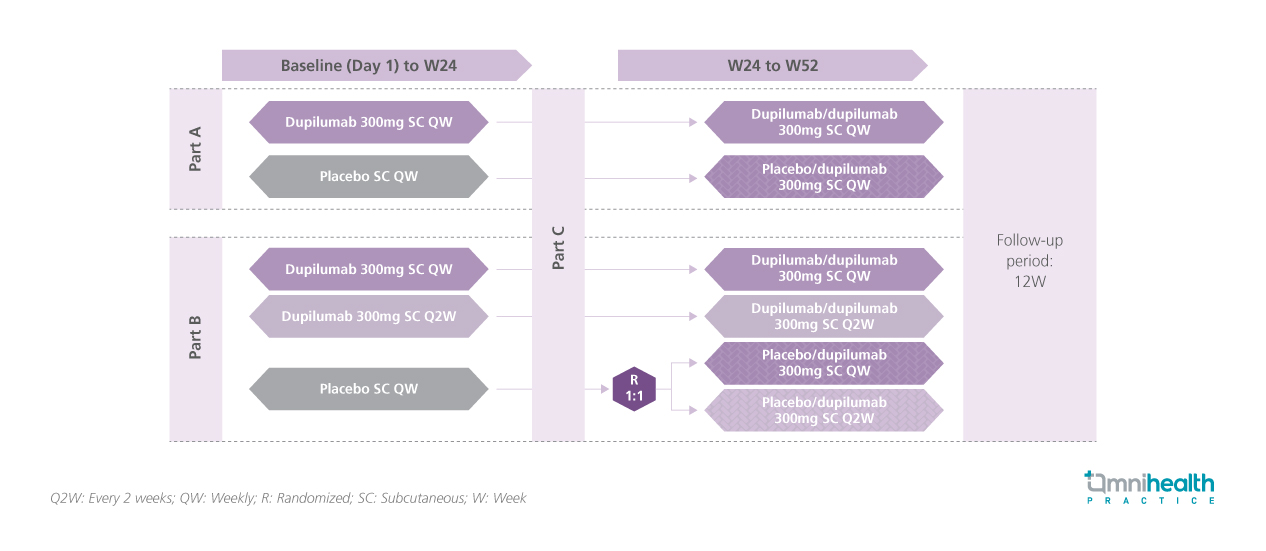
STUDY DESIGN
Eosinophilic esophagitis (EoE) is a chronic, progressive, type 2 inflammatory disease of the esophagus.1 While food elimination diets (FED) are an established treatment for EoE, their impact on the efficacy of medical therapy remains unclear.1 Dupilumab, approved in the United States and European Union for patients aged ≥1 year and weighing ≥15kg, offers a targeted treatment option.1 A post hoc analysis of the LIBERTY EoE TREET trial evaluated whether the efficacy of once-weekly (QW) dupilumab differs in adolescents and adults with or without concurrent FED.1 This post-hoc analysis included patients from parts B and B-C of the phase 3 LIBERTY EoE TREET trial.1 In part B, patients received dupilumab 300mg or placebo weekly until week 24 (W24), while those continuing into part C received dupilumab 300mg weekly until W52.1 At screening, 60 patients (38.0%) were following FED and were instructed to maintain it throughout the study.1 The key efficacy outcomes assessed, including histologic remission (≤6 eosinophils per high-power field [eos/hpf]) and absolute mean changes from baseline in Dysphagia Symptom Questionnaire (DSQ) score, Endoscopic Reference Score (EREFS), and EoE Histologic Scoring System (EoE-HSS) grade/stage, were analyzed based on concurrent FED status at W24 and W52.1
“Dupilumab QW improved histologic, symptomatic, and endoscopic features of EoE in adolescents and adults up to 24 weeks vs. placebo, irrespective of concurrent FED. Improvements were sustained up to 52 weeks"
Dr. Antonella Cianferoni
The Children’s Hospital of Philadelphia, Pennsylvania, United States
FINDINGS
|
Efficacy endpoints: |
|
|
| Safety: |
|

