MEETING HIGHLIGHT
Bringing immunotherapy to the forefront of NPC treatment with tislelizumab
With nasopharyngeal carcinoma (NPC)’s high prevalence in Southeast Asia and its significant public health implications, the need for effective treatment options is critical.1 At a recent lecture, Professor Tang, Ling-Quan from the Sun Yat-Sen University Cancer Center discussed the current landscape of immunotherapy for NPC. The lecture highlighted the limitations of traditional therapies, the promising role of immunotherapy, particularly with programmed cell death protein 1 (PD-1) inhibitors such as tislelizumab*, and key findings from pivotal studies such as RATIONALE-309 and BEACON.2,3 These insights underscore the evolving strategies aimed at improving patient outcomes in locoregionally advanced NPC (LANPC).
The current landscape of immunotherapy for LANPC
NPC is highly prevalent in Southeast Asia and southern China, with over 51,000 new cases diagnosed in China in 2022, highlighting its significance as a public health issue.1 The development of NPC is influenced by genetic predisposition, environmental factors, and the Epstein-Barr virus (EBV), which plays a critical role in tumor formation.2 Notably, more than 70% of NPC patients are diagnosed at the locoregionally advanced stage, underscoring the urgent need for effective early-stage treatment.4 Current first-line treatment typically involves chemotherapy and radiotherapy, which may not always yield optimal outcomes.2 This has prompted a growing interest in immunotherapy as a promising option.1 PD-L1 expression in the tumor microenvironment suggests potential responsiveness to immune checkpoint inhibitors, paving the way for innovative treatment strategies.5 Prof. Tang emphasized the evolving role of immunotherapy in managing LANPC, aiming to enhance patient outcomes. Tislelizumab is an anti-PD-1 monoclonal antibody that inhibits the PD-1 checkpoint protein, enhancing T-cell activation and allowing the immune system to better recognize and attack tumors.2 Engineered to reduce binding to Fcγ receptors and minimize antibody-dependent cellular phagocytosis (ADCP), it has high affinity and specificity for PD-1, demonstrating antitumor efficacy in various tumor types.2 Clinical studies, including RATIONALE-309 and BEACON, have demonstrated the promising efficacy of tislelizumab, leading to improved progression-free survival (PFS) and overall survival (OS) rates in patients with NPC, especially when used in combination with chemotherapy.2,3
Immunotherapy breakthrough: RATIONALE-309 study results for R/M NPC
The RATIONALE-309 is a pivotal randomized controlled trial aimed at assessing the efficacy of tislelizumab in combination with chemotherapy for patients with recurrent/metastatic (R/M) NPC.2 Patients were randomly assigned 1:1 to one of two groups: the test group receiving tislelizumab 200mg intravenously (IV) every 3 weeks (Q3W) + chemotherapy (gemcitabine + cisplatin [GP]) and the control group receiving placebo Q3W+ chemotherapy (GP).2 Patients were allowed to crossover after disease progression.2
At a median follow-up of 15.5 months, the median PFS was 9.6 months for the tislelizumab group, compared to 7.4 months for the placebo group, indicating a 50% lower risk of disease progression or death (HR=0.50; 95% CI: 0.37-0.68; to evaluate p<0.0001).2 OS also improved significantly in the tislelizumab group, with the median OS not reached for the tislelizumab group and 23.0 months for the placebo group, reflecting a 40% lower risk of death (HR=0.60; 95% CI: 0.35-1.01).2
In an earlier analysis presented at the American Society of Clinical Oncology (ASCO) Annual Meeting 2022, there was a significant improvement in the time from randomization to tumor assessment of progression on next-line (second-line) therapy or death due to any cause, whichever came first (PFS2) for the tislelizumab + chemotherapy group, with a HR of 0.38 (95% CI: 0.25-0.58), indicating a 62% reduction in the risk of disease progression or death compared to the control group.6 The median PFS2 was not reached for the tislelizumab group, while it was 13.9 months for the placebo group.6 The probability of achieving PFS2 was notably higher in the tislelizumab group at various time points: 95.3% at 6 months, 93.6% at 9 months, and 83.4% at 12 months.6 This significant reduction in PFS2 risk highlights the efficacy of incorporating immunotherapy early in the treatment regimen for R/M NPC (figure 1).6
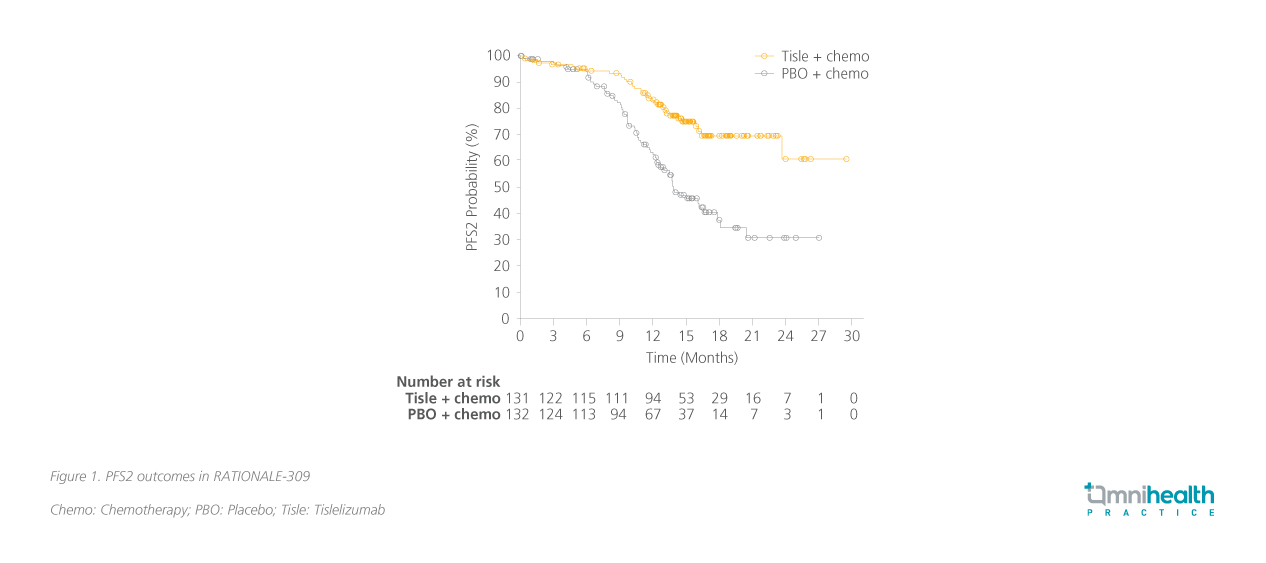
The safety profile of tislelizumab + chemotherapy was manageable and consistent with earlier findings, showing no new safety concerns.6 Serious adverse reactions and those leading to treatment discontinuation, as well as immune-mediated adverse reactions, were infrequent, highlighting a favorable safety profile.6 The RATIONALE-309 study provides evidence supporting the use of tislelizumab in combination with chemotherapy for patients with R/M advanced NPC.2,6 The significant improvement in PFS2 outcomes highlights that tislelizumab combined with chemotherapy is superior to second-line rescue therapy, offering longer-lasting benefits when used in first-line treatment.2
Moving the use of immunotherapy earlier for LANPC
In view of the impressive results of immunotherapy in the R/M setting, several studies have been conducted on its use in earlier-line treatment. Prof. Tang summarized them into multiple treatment modalities, including the continuous modality, which combines induction therapy, radiotherapy, and adjuvant therapy for comprehensive tumor targeting; the sandwich modality, which involves induction therapy followed by adjuvant therapy to consolidate treatment effects; the induction modality, which focuses solely on reducing tumor burden before further treatment; and the adjuvant modality, used after primary treatment to prevent recurrence. Prof. Tang proceeded to provide an overview of some ongoing studies including the DIAMOND study which investigated toripalimab combined with de-intensification radical chemoradiotherapy in LANPC as well as the CONTINUUM study which examined the efficacy of sintilimab as an induction therapy combined with chemoradiotherapy. These findings have set the stage for the use of tislelizumab in earlier-line treatments, as investigated in the BEACON study.3
The BEACON study: Significant improvement in CR rate with tislelizumab
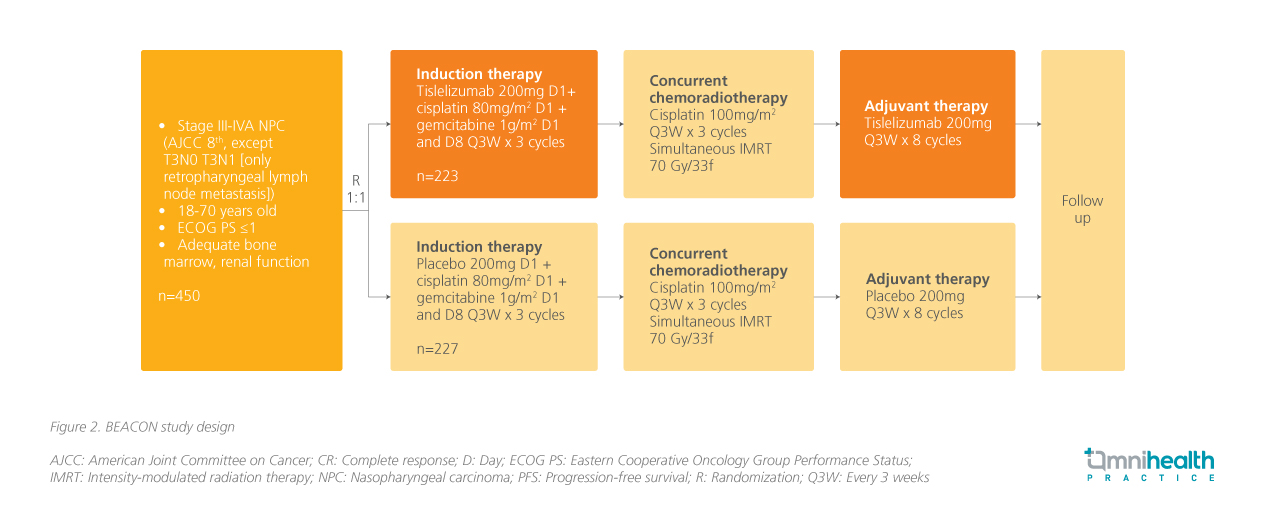
The BEACON study, a randomized double-blind phase 3 trial, aimed to evaluate the efficacy and safety of tislelizumab + GP for patients with LANPC (figure 2).3 The enrolled 450 patients with stage III-IVa NPC (excluding T3N0 and T3N1 with retropharyngeal lymph node metastasis) and involved a test group receiving tislelizumab + GP for three cycles, while the control group received a placebo + GP for the same duration.3 Following this, all participants received concurrent chemoradiotherapy (CCRT) with cisplatin for three cycles, followed by additional cycles of tislelizumab or placebo.3
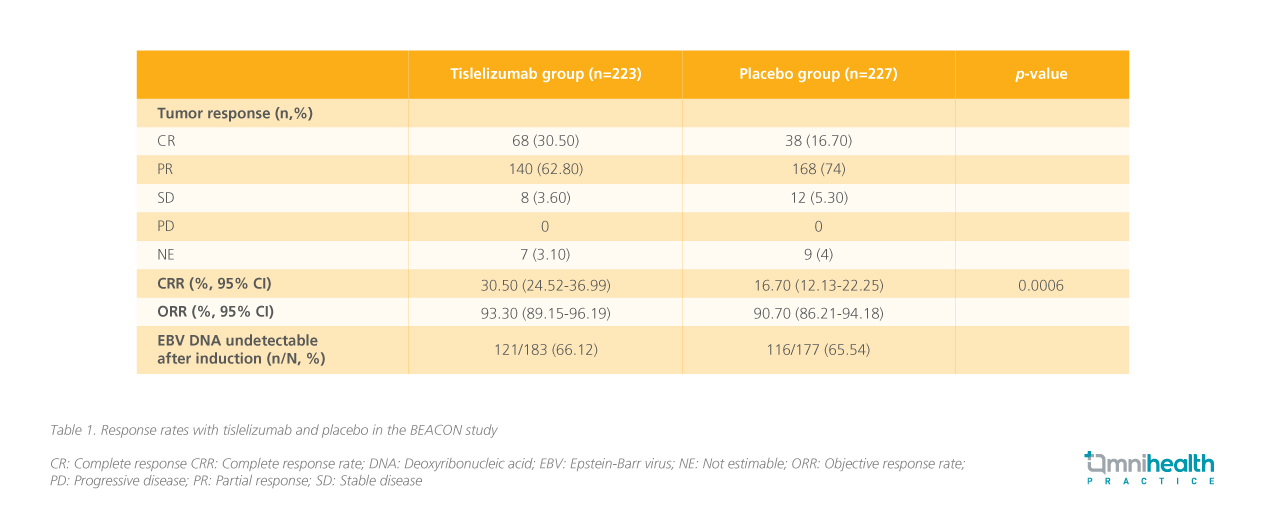
The primary endpoint was complete response rate (CRR) which was 30.50% in the tislelizumab group compared to 16.70% in the placebo group, while the overall response rate (ORR) was 93.3% vs. 90.7%, respectively (table 1).3
Induction therapy with tislelizumab + GP was generally well tolerated, demonstrating a manageable safety profile.3 In the safety population (tislelizumab group: n=219; placebo group: n=224), the incidence of grade ≥3 treatment-emergent adverse events (TEAEs) was similar between the two groups (40.6% vs. 39.3%), as was the rate of serious adverse events (SAEs) (2.3% vs. 1.3%).3
CSCO guideline recommendations affirm the role of PD-1 inhibitors
In light of these data, the Chinese Society of Clinical Oncology (CSCO) has updated its guidelines for managing NPC.7 The guidelines recommend a combination of chemotherapy - typically using GP - paired with immunotherapy, such as PD-1 inhibitors, as the standard first-line treatment.7 Additionally, the use of adjuvant immunotherapy, particularly PD-1 inhibitors, following concurrent chemoradiotherapy is advised to further enhance patient outcomes and reduce the risk of recurrence.7 Prof. Tang emphasized that the combination of PD-1 inhibitors with GP has now become the standard treatment regimen for R/M NPC. Data from phase 3 clinical studies, including BEACON, have demonstrated the efficacy of PD-1 inhibitors in treating LANPC, supporting their early use in patients.3 Ongoing immunotherapy trials are exploring optimal treatment modalities to maximize efficacy and improve patient care.
Conclusion
In conclusion, the advancements in immunotherapy, particularly the use of tislelizumab, are transforming the treatment landscape for NPC. With significant improvements in PFS and OS rates demonstrated in pivotal studies like RATIONALE-309 and BEACON, positioning immunotherapy as a vital component of first-line treatment protocols.2,3 As guidelines evolve to incorporate these innovative strategies, the focus on early intervention with PD-1 inhibitors holds promise for enhancing patient outcomes.2 Continued research and clinical trials will be essential in refining treatment approaches and ultimately improving survival rates for patients with advanced NPC.
*Tislelizumab has not been approved for NPC in Hong Kong as of the date of publication

