MEETING HIGHLIGHT
Evolving insights in Asian R/M NPC care: A case sharing of tislelizumab
Nasopharyngeal carcinoma (NPC) disproportionately affects populations in Southeast Asia (SEA), leading to a heightened disease burden in these regions.1 To address the unique needs of this patient population, organizations such as the Chinese Society of Clinical Oncology (CSCO) provide tailored clinical practice guidelines.2 These guidelines incorporate the latest data from recent clinical trials, highlighting the role of immune checkpoint inhibitors (ICIs) in the management of NPC.2 At the Beacon 2.0 Forum hosted by the Best IO Academy, Dr. Sumou, Ingrid Karmane, one of the guest speakers, outlined the CSCO recommendations along with the key findings from the phase 3 RATIONALE-309 trial. It demonstrated improvements in progression-free survival (PFS) with tislelizumab + chemotherapy compared to placebo + chemotherapy in patients with recurrent/metastatic (R/M) NPC, whilst also noting tislelizumab’s favorable safety profile.3 The presentation was supplemented with a case study illustrating the use of tislelizumab, and a discussion session by the attending physicians.
Elevated incidence and impact of NPC in the Asian regions
The majority of NPC patients, over 70%, present with locoregionally advanced stage disease.4 This challenging clinical landscape is further complicated by the high incidence of NPC in SEA regions.1 Dr. Sumou stated, “Over 70% of new cases diagnosed are in SEA regions. China alone makes up almost 50% of global new NPC cases.” Although the addition of gemcitabine and cisplatin induction chemotherapy (GPIC) has led to improvements in prognosis, roughly 20% of patients still develop recurrence or metastases.5 The substantial disease burden and high number of NPC patients in these Asian regions render it an important public health issue that requires focused attention and intervention.1
The rising prominence of immunotherapy in NPC
Over the past decade, extensive research has been conducted to better understand the mechanisms of NPC and to explore treatment options.6-8 ICIs such as inhibitors of programmed cell death protein 1 (PD-1) and its ligand programmed cell death ligand-1 (PD-L1) have also independently earned an increasingly prominent role in cancer therapy.6 The tumor microenvironment of NPC is characterized by heavy infiltration of immune cells, and most NPC tumor cells express the immunosuppressive protein PD-L1, suggesting that immune-related factors play an important role in the development of NPC, prompting research into the use of PD-1 and PD-L1 inhibitors as potential treatment options.7 These observations have led to investigations into the therapeutic potential of ICIs, as a means of harnessing the body's natural immune response against the cancer.7
Large RCTs such as the phase 3 RATIONALE-309 trial have shown that adding PD-1 inhibitors to the GC chemotherapy backbone is effective as a first-line treatment for R/M NPC.3 As a result, chemoimmunotherapy is now listed as the first-line treatment of R/M NPC by both international and CSCO guidelines across a broad range of patients regardless of surgery or radiotherapy eligibility in patients with local recurrences.2 Tislelizumab is one of these immunotherapy options listed in the CSCO guidelines.2 It is a uniquely designed immunoglobulin G4 (IgG4) anti-PD-1 monoclonal antibody (mAb) and has emerged as one of the novel immunotherapy options against R/M NPC.8 The phase 3 RATIONALE-309 study investigated the efficacy and safety of tislelizumab + chemotherapy compared to placebo + chemotherapy and had already generated positive results in the latest interim analyses over a median follow-up of 15.5 months.3
A closer look at tislelizumab in RATIONALE-309
The RATIONALE-309 studied the efficacy and safety of tislelizumab in treatment-naïve adult patients with R/M NPC (n=263).3 Patients had histologically or cytologically confirmed R/M NPC with ≥1 measurable lesion and an Eastern Cooperative Oncology Group-Performance Score (ECOG-PS) ≤1.3 They were randomized 1:1 to receive intravenous (IV) tislelizumab 200mg every 3 weeks (Q3W) (n=131) or a placebo (n=132) on top of chemotherapy, stratified by gender and the presence of liver metastasis.3 The chemotherapy regimen was gemcitabine 1g/m² IV on day 1 and day 8, and cisplatin 80mg/m² IV on day 1.3 This regimen was administered every 3 weeks for 4-6 cycles, with treatment continuing until disease progression, intolerable toxicity, death, or withdrawal of consent.3 The tislelizumab group was allowed to continue tislelizumab monotherapy, and the placebo group was allowed to crossover to receive the same regimen after disease progression.3
Almost half (49.2%) of patients in the placebo arm crossed over to receive tislelizumab monotherapy after disease progression.3 At the interim analysis with a median follow-up of 15.5 months, the PFS and PFS after the next-line of therapy (PFS2) data have offered insights into the therapeutic efficacy of tislelizumab.3
Unpacking the efficacy and safety of tislelizumab
The primary endpoint was PFS where tislelizumab + chemotherapy led to a significant 50% risk reduction in progression or death over placebo + chemotherapy (HR=0.50; 95% CI: 0.37-0.68; p<0.0001) (figure 1).3 The median PFS was 9.6 months and 7.4 months in the tislelizumab and placebo arms, respectively.3 Results were consistent across different levels of PD-L1 expression and Epstein-Barr virus (EBV) deoxyribonucleic acid (DNA) level.3 The study also reported a 61% risk reduction in PFS2 with the median PFS2 not reached vs. 16.6 months in the placebo arm.3 Although the PFS2 and overall survival (OS) data were immature at the time of cutoff, Dr. Sumou noted a clear benefit across both measures with a favorable trend toward tislelizumab.3
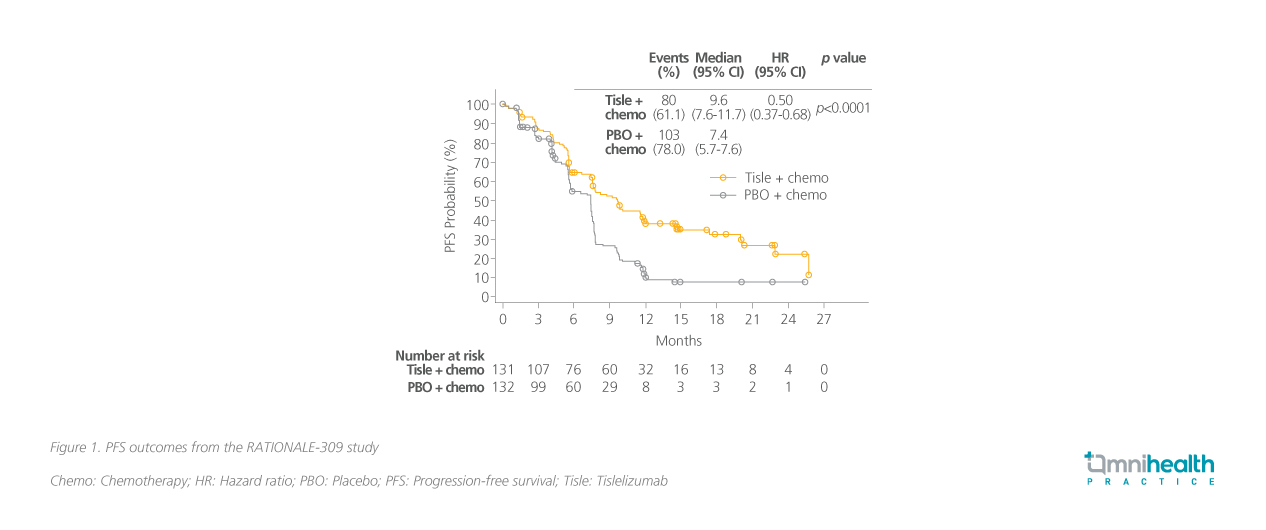
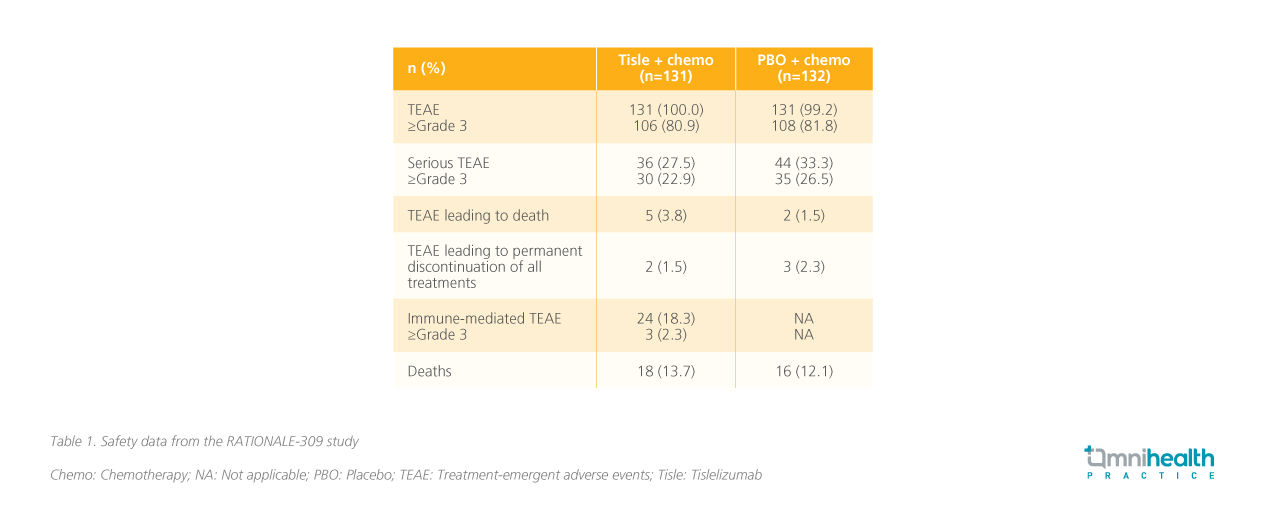
A closer look at the safety outcomes in RATIONALE-309 revealed that the safety profile of tislelizumab was manageable and consistent with previous reports (table 1).3 With the addition of immunotherapy, the rates of immune-mediated adverse events were also low and were mostly manageable.3 Hypothyroidism was noted as the most common (13.7%) immune-mediated adverse event, but Dr. Sumou stated that it was a blessing that more severe immune-mediated adverse events such as myocarditis were rare.
Following the data from RATIONALE-309, Dr. Sumou proceeded to share her experience with tislelizumab in a case of R/M NPC where rapid resolution of NPC was achieved after only 3 cycles of tislelizumab treatment.
A case study of tislelizumab in NPC management
The patient was a 64-year-old male with a height of 156cm and a body weight of 47kg. In May 2023, he presented with a 1-month history of a painless right neck lump, right ear fullness, and hearing loss. He was a non-smoker. Physical examination revealed enlarged, hard, and poorly mobile lymph nodes (approximately 1.5cm) in the right neck region (levels II/III), without tenderness. Fiberoptic endoscopy of the nasopharynx showed a tumor involving the right posterior parietal wall, and the right tympanic membrane was retracted with effusion.
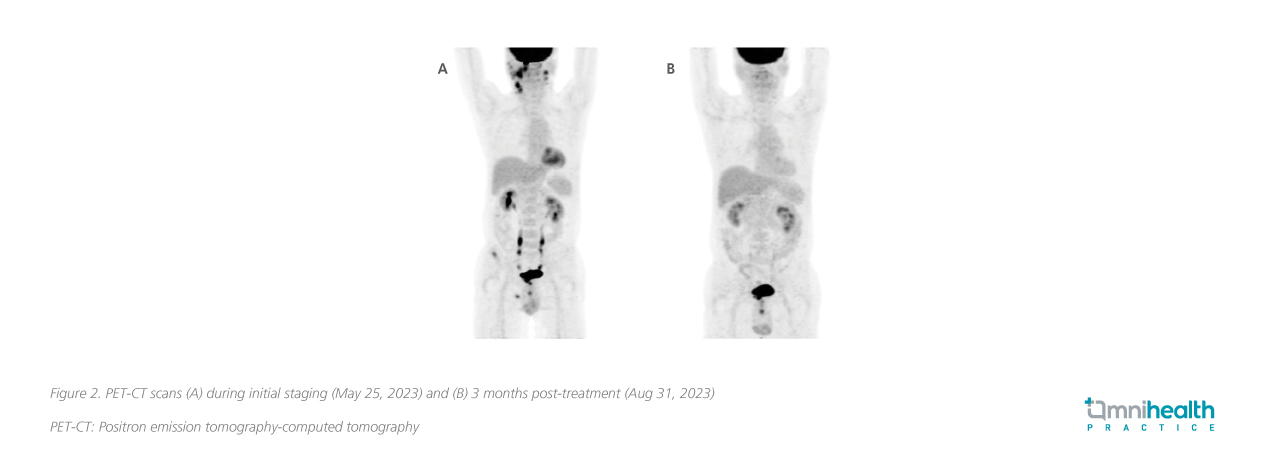
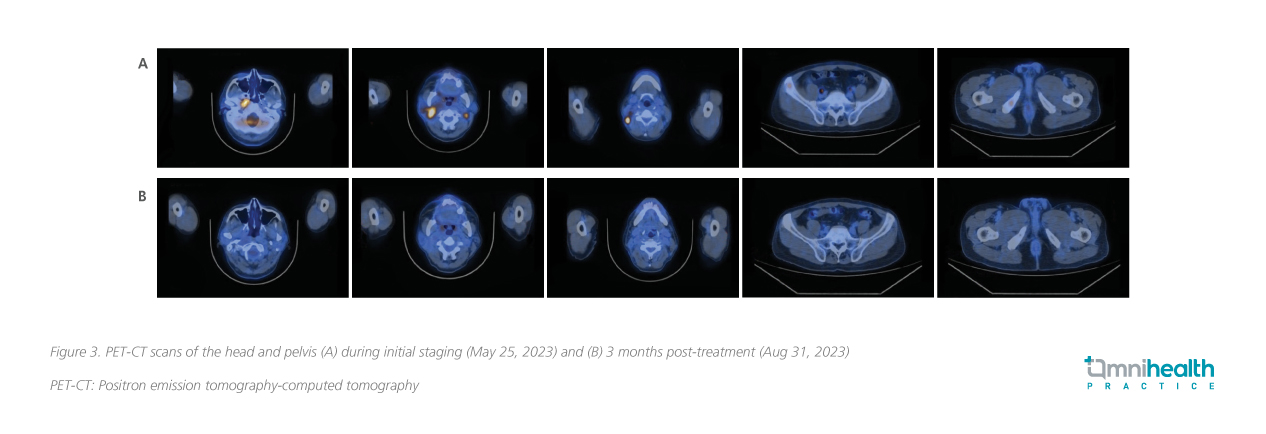
Biopsy of the nasopharyngeal tumor confirmed a diagnosis of NPC, an undifferentiated non-keratinizing subtype. Positron emission tomography-computed tomography (PET-CT) revealed a hypermetabolic mass at the right nasopharynx consistent with NPC, with multiple lymphatic metastases in bilateral retropharyngeal spaces, bilateral level II, right level III, right level V of neck, right parotid gland, and bone metastasis in the right iliac wing and right ischium, with clinical staging of cT3N2M1, stage IVB (figure 2A, 3A). The baseline plasma EBV-DNA level was 274 IU/mL. He tested negative for the hepatitis B surface antigen (HBsAg). No genomic alterations of clinical actionability were identified and he was also homologous recombinant deficiency (HRD)-negative.
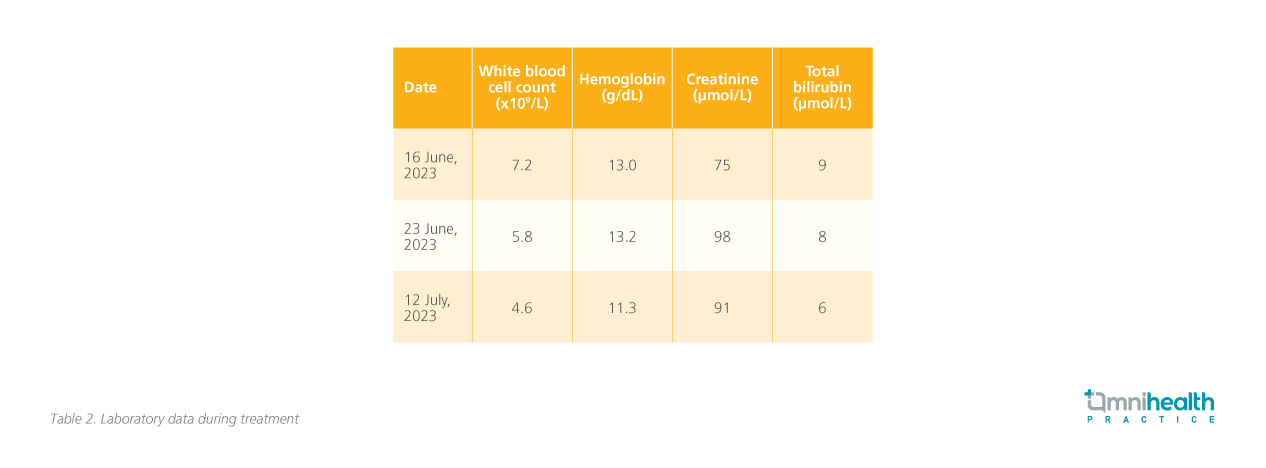
The patient was prescribed the RATIONALE-309 regimen and received 3 cycles of this treatment on June 17, July 13, and Aug 3, 2023. To manage the potential side effects of chemotherapy, supportive measures including antiemetics and corticosteroids were provided. The patient also received pre-radiotherapy dental management, as well as nursing and health education related to radiotherapy and chemotherapy. The patient was also provided with exercise and diet counseling. After 3 cycles of treatment, there was notable improvement. Response evaluation by PET-CT in Aug 2023 showed resolution of the nasopharyngeal tumor, as well as almost complete remission of the metastatic lymphadenopathies and bone metastasis (figure 2B, 3B). Plasma EBV-DNA dropped to an undetectable level. Overall, the treatment was well-tolerated with manageable hematological side effects (table 2).
Volumetric Modulated Arc Therapy (VMAT) with 70Gy/30frs irradiation was delivered to the nasopharyngeal tumor and enlarged lymph nodes on both sides of the neck, as well as 66Gy/frs to the above lesions plus about 0.3cm-0.5cm margin, 60Gy/30frs to high- risk regions in the head and bilateral upper neck, 54Gy/30frs to the bilateral lower neck and supraclavicular fossae lymph drainage areas, then boost 4.6Gy/2frs to the residual nasophar yngeal tumor. Concurrent chemotherapy with cisplatin 30mg/m2-35mg/m2 was arranged for 5 cycles and then stopped due to intolerance. CCRT was per formed from Sept 21 to Nov 7, 2023. He suf fered from mild to moderate radiation- induced acute mucositis and dermatitis .
Post-treatment fiberopt ic endoscopy in Jan 2024 showed a smooth nasophar ynx, and absence of tumoral lesion. Tislelizumab monotherapy was resumed. Surveillance magnetic resonance imaging (MRI) in Apr 2024 showed no R/M tumor. Fiberoptic endoscopy in May 2024 did not show any new lesions. Plasma EBV-DNA level remained undetectable. The patient was able to continue his usual activities and repor ted high sat isfact ion with the treatment.
A case study of tislelizumab in NPC management
The patient was a 64-year-old male with a height of 156cm and a body weight of 47kg. In May 2023, he presented with a 1-month history of a painless right neck lump, right ear fullness, and hearing loss. He was a non-smoker. Physical examination revealed enlarged, hard, and poorly mobile lymph nodes (approximately 1.5cm) in the right neck region (levels II/III), without tenderness. Fiberoptic endoscopy of the nasopharynx showed a tumor involving the right posterior parietal wall, and the right tympanic membrane was retracted with effusion.
Key takeaways from the panel dialogue
The forum concluded with a panel discussion that reviewed the improvement s seen in the case study. Detailed evaluations are crucial to guide personalized treatment planning for NPC. Prof. Mai Hai-Qiang from the Sun Yat-sen University Cancer Center stated, “If tests for high expression of immune cells are feasibly conducted, it would bet ter guide the efficacy of immunotherapy, and patients would be more willing to accept maintenance therapy.” Regarding the case, Prof. Li Zhi-Xin from the Capital Medical University affiliate Beijing Tongren Hospital acknowledged that “whether three cycles of chemotherapy is sufficient and whether we need to intensify treatment in the later auxiliary phase is still open for discussion.” The panelists recognized the transformative potential of immunotherapy, with the current standard including immunotherapy combined with chemotherapy as the first-line therapy for R/M NPC. Immunotherapy may be more effective than traditional chemotherapy, especially for patients with high immune cell expression, as Prof. Mai further stated that “knowing its carryover effects of immunotherapy, the old model must be updated.” Prof. Li emphasized that “Unlike induction therapy, our goal is to extend survival and improve the quality of life.” Thorough baseline assessment s and a balance between treatment intensity and pat ient quality of life are impor tant considerations. Delaying radiotherapy could be beneficial for patient s who achieve good disease control with immunotherapy. Ongoing research is needed to refine s trategies and optimize outcomes for this challenging disease.
Conclusion
The management of NPC in SEA populations remains a critical public health concern, which the CSCO guidelines aim to address with immunotherapies, such as tislelizumab, based on the latest clinical evidence.1,2 Dr. Sumou highlighted the remarkable outcomes observed with tislelizumab, underscoring its transformative potential in NPC management, not just in terms of its high ef ficacy in delaying progression and prolonging survival, but also in terms of it s favorable safety profile.3 The case study and discussion session reinforced the practical application of these evidence-based guidelines, fostering collaborative exchange to optimize care and outcomes for patient s in SEA.

