MEETING HIGHLIGHT
Pinnacle 4-year data from OPTIC supports durable efficacy of ponatinib in CP-CML with BCR::ABL1 T315I mutation
Despite the availability of first- and second-generation tyrosine kinase inhibitors (TKIs), patients with chronic phase chronic myeloid leukemia (CP-CML) harboring the BCR::ABL1 T315I mutation continue to face poor survival outcomes, often due to the development of resistance.1 Ponatinib, a third-generation TKI, has previously demonstrated significant efficacy in treating patients with CP-CML regardless of BCR::ABL1 mutation status.2-4 At the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Professor Michael Deininger presented key findings from the 4-year follow-up post-hoc analysis of the OPTIC trial, an open-label phase 2 study investigating the efficacy, safety and dose-response relationship of ponatinib in CP-CML patients with the difficult-to-treat T315I mutation.2,5
Unmet need in the T315I CP-CML treatment landscape
CML typically presents in the chronic phase (CP) and progresses to more advanced, treatment-resistant stages without effective intervention.1 While the adoption of firstand second-generation TKIs has conferred clinical benefits in patients with CP-CML, intolerance and resistance have contributed to suboptimal survival outcomes.1 In particular, patients with the BCR::ABL1 T315I mutation have a worse prognosis, especially in terms of overall survival (OS), progression-free survival (PFS) and failure-free survival compared to those without the mutation.1 As such, there is a need for novel, well-tolerated therapies that can induce durable responses and improve outcomes for patients with T315I CP-CML.1
Ponatinib, a novel alternative for managing BCR::ABL1 T315I-mutated CP-CML
Ponatinib is a third-generation TKI that can inhibit native BCR::ABL1 and all known single-resistance mutations, including the difficult-to-treat T315I.2 This has enabled ponatinib to earn its endorsement by international guidelines, including the 2020 European LeukemiaNet (ELN) guidelines and the 2017 European Society for Medical Oncology (ESMO) guidelines, for treating CP-CML patients who have failed prior treatment approaches with earlier generation TKIs.3,4
To assess and optimize the efficacy of ponatinib, the phase 2 OPTIC trial recruited patients with CP-CML who were resistant to ≥2 prior TKIs or harboring the T315I mutation and employed a response-based dosing strategy, where patients were randomized into 3 cohorts with starting daily doses of 45mg (n=94), 30mg (n=95), and 15mg (n=94) of ponatinib respectively.2 The primary endpoint was achieving complete cytogenetic response (CCyR), defined as a BCR::ABL1 transcript level ≤1% (BCR::ABL1IS) at 12 months.2 Upon achievement of the primary endpoint, patients in the 45mg and 30mg cohorts were required to reduce their subsequent dose to 15mg.2 Dose reduction to a minimum of 10mg was allowed for patients who experienced adverse events (AEs).2
Results from the OPTIC trial highlighted ponatinib’s efficacy in facilitating a CCyR rate ranging from 44.1%-23.1% after 12 months. 2 At the primary analysis with a median follow-up of 32 months, ponatinib demonstrated promising efficacy in the overall CP-CML population as well as in the high-risk BCR::ABL1 T315I subgroup, with the estimated 36-month PFS and OS ranging between 66%-73% and 89%-92% respectively.6 During the 2024 ASCO Annual Meeting, Prof. Deininger presented the key results from a 4-year follow-up post-hoc analysis of the OPTIC trial, where the BCR::ABL1IS response rates, PFS, and OS in patients with the T315I mutation were reported.5
Characterizing the 4-year efficacy of ponatinib in OPTIC
The 4-year efficacy outcomes of the OPTIC trial exhibited ponatinib’s durable efficacy, particularly in patients with the T315I mutation.5 At 48 months, those who received the 45mg starting dose showed greater response rates compared to patients who started on lower doses, particularly in achieving ≤1% BCR::ABL1IS (figure 1).5
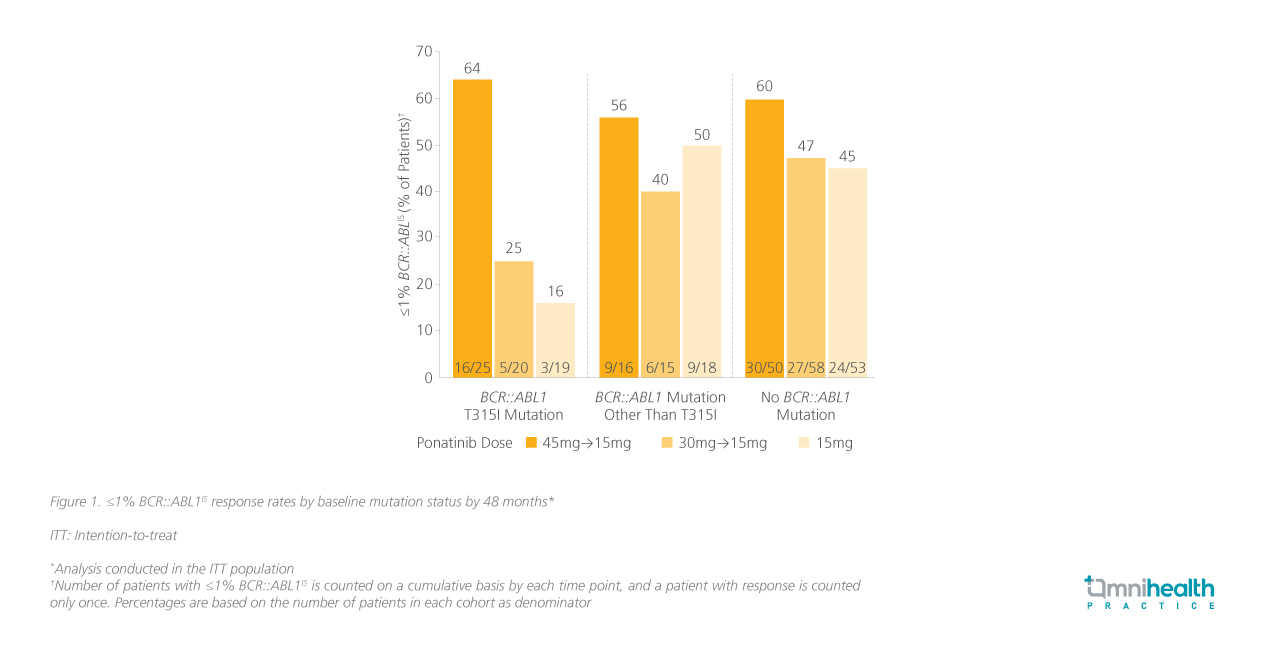
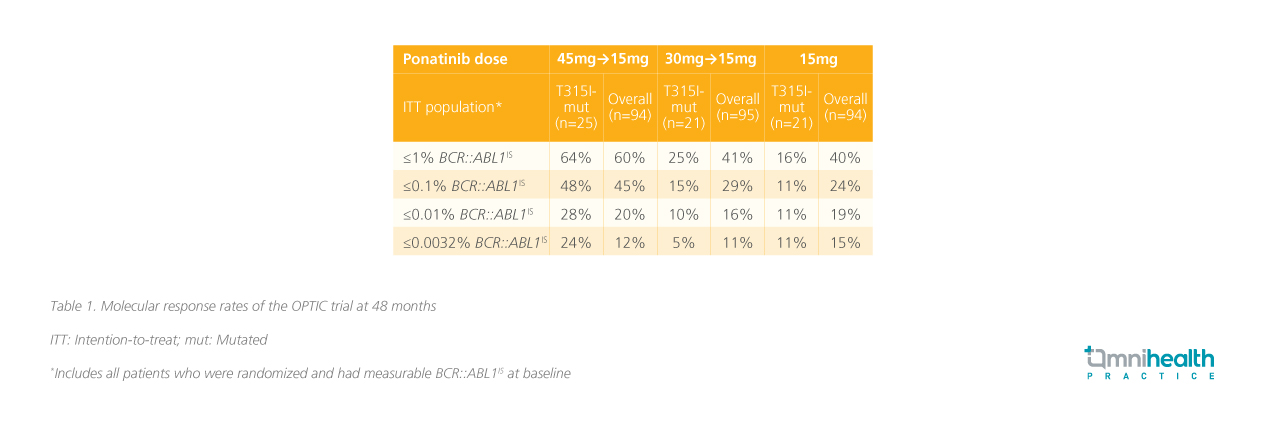
In the trial, the proportions of patients with the BCR::ABL1 T315I mutation were well-balanced in the 3 dosing cohorts (45mg→15mg: 27%; 30mg→15mg: 22%; 15mg: 22%).5 By 48 months, the proportion of patients with the T315I mutation achieving ≤1% BCR::ABL1IS was the highest in the 45mg cohort (64%) compared to those who started on lower daily doses of 30mg (25%) and 15mg (16%).5 The difference in response rates between the dosing cohorts was the highest in the T315I-mutated cohort.5 Similar trends were seen when other BCR::ABLIS cutoffs were used (table 1).5
In terms of survival outcomes, median PFS was not reached (NR), 28.4 months and 45.6 months in the 45mg, 30mg and 15mg cohorts, respectively.5 At 4 years, patients with the T315I mutation who started on the 45mg dose had a higher rate of PFS (75%) compared to those who started on lower doses (30mg: 40%; 15mg: 41%), indicating a better long-term disease control (table 2).5 On the other hand, median OS was NR at the analysis, regardless of mutation status across all dosing cohorts (table 2).5
The manageable safety profile of ponatinib
The safety results of the OPTIC trial affirmed the long-term tolerability of ponatinib and its responsebased dose-modification regimen.5 The analysis reported treatment-emergent adverse events (TEAEs), with a focus on grade 3-4 events.5 A preponderance of these events in the 45mg cohort was observed, which was expected given the higher starting dose.5 Safety outcomes of special interest in the study included the rates of treatment-emergent arterial occlusive events (TE-AOEs), which were generally low across the dosing cohorts regardless of mutation status, with none leading to death.5
In the 45mg cohort, 15 patients with T315I mutation had reached the primary endpoint and underwent dose reductions, of which 47% (n=7/15) maintained their response after the dose reduction.5 8 patients had their doses re-escalated due to loss of response, of which 75% (n=6/8) regained ≤1% BCR::ABL1IS after re-escalation.5
Conclusion
The 4-year follow-up data from the OPTIC trial supported ponatinib's sustained efficacy and manageable safety profile in CP-CML patients with T315I mutation. The analysis revealed that starting patients on a 45mg dose of ponatinib led to higher response rates and prolonged PFS. The safety profile of ponatinib was generally manageable across the different dose levels evaluated, with a low incidence of TE-AOE. These findings underscore the important role ponatinib can play in improving outcomes for patients with CP-CML who are resistant to second-generation BCR::ABL1 TKI therapy, including those with the challenging T315I genetic alteration.

