CONFERENCE UPDATE: ASCO 2024
Phase 3 PhALLON trial reveals high rates of MRD negativity at any time and prolonged PFS with ponatinib in patients with newly diagnosed Ph+ ALL
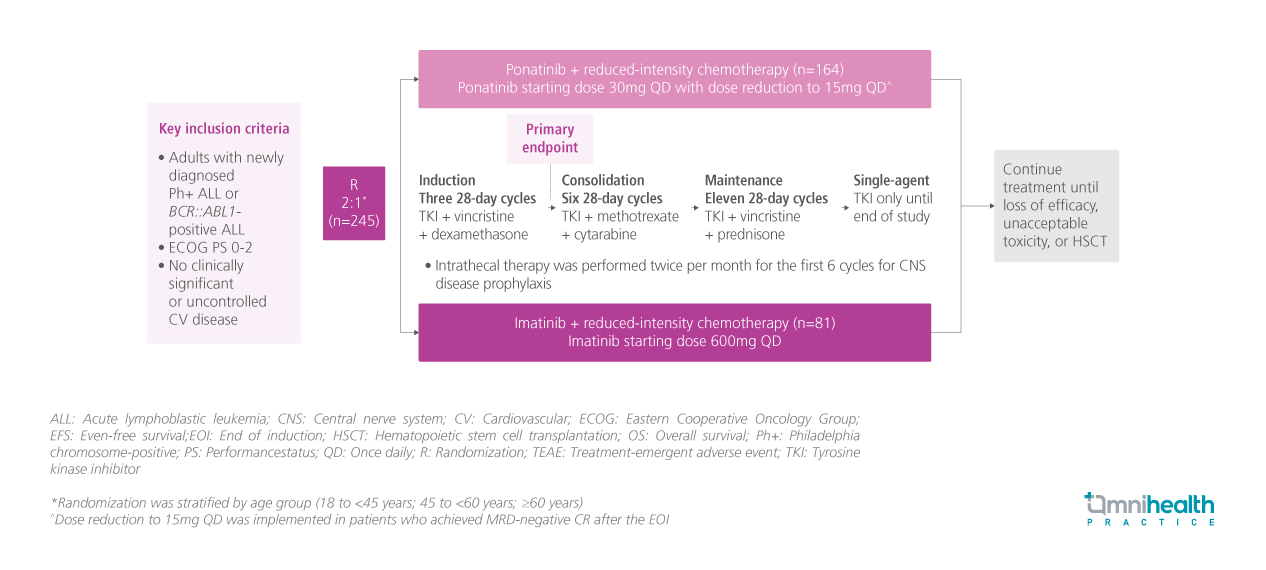
STUDY DESIGN
Ponatinib is a third-generation BCR::ABL tyrosine kinase inhibitor (TKI) that inhibits BCR::ABL1 regardless of any single resistance mutations, including T315I.1 It was previously evaluated in the PhALLCON (ponatinib-3001) trial in adults with newly diagnosed Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL).1 The trial met its primary endpoint with a significantly higher rate of minimum residual disease (MRD)-negative complete remission (CR) at the end of induction (EOI) with ponatinib, compared with imatinib (34.4% vs. 16.7%; p=0.002).1
PhALLCON is a global, phase 3, randomized, open-label, multicenter trial conducted to compare the efficacy and safety of ponatinib and imatinib in newly diagnosed Ph+ ALL.1 A total of 245 eligible patients were randomized 2:1 to receive ponatinib starting dose of 30mg once daily (QD) (n=164) or imatinib 600mg QD (n=81) in combination with 20 cycles of reduced-intensity chemotherapy.1 In the ponatinib arm, a dose reduction to 15mg QD was offered when MRD-negative CR was achieved after the EOI.1
The captioned post-hoc subgroup analysis of PhALLCON evaluated the rates of MRD negativity and progression-free survival (PFS) in randomized patients with p190/p120 BCR::ABL1 variants confirmed by a central laboratory (ponatinib n=154; imatinib n=78) over a median trial period of 20.1 months.1 These efficacy outcomes were stratified by age (<65 and ≥65 years), and BCR::ABL1 variant subgroups (p190 and p210).1 MRD negativity was defined as BRC::ABL1IS ≤0.01%.1 PFS events included any-cause death, failure to achieve CR by EOI, relapse from CR, and failure to achieve or loss of MRD negativity.1 Outcomes in patients who proceeded to hematopoietic stem cell transplantation (HSCT) were also explored.1 TEAEs, including arterial occlusive events (AOEs) and venous thromboembolic events (VTEs), were evaluated in all patients and those without HSCT.1
FINDINGS
| Efficacy outcomes: |
|
|
|
|
|
Safety: |
|
|

