CONFERENCE UPDATE: ESC 2024
Ticagrelor alone after drug-eluting coronary stenting halves major bleeding risks in PCI patients regardless of ACS status
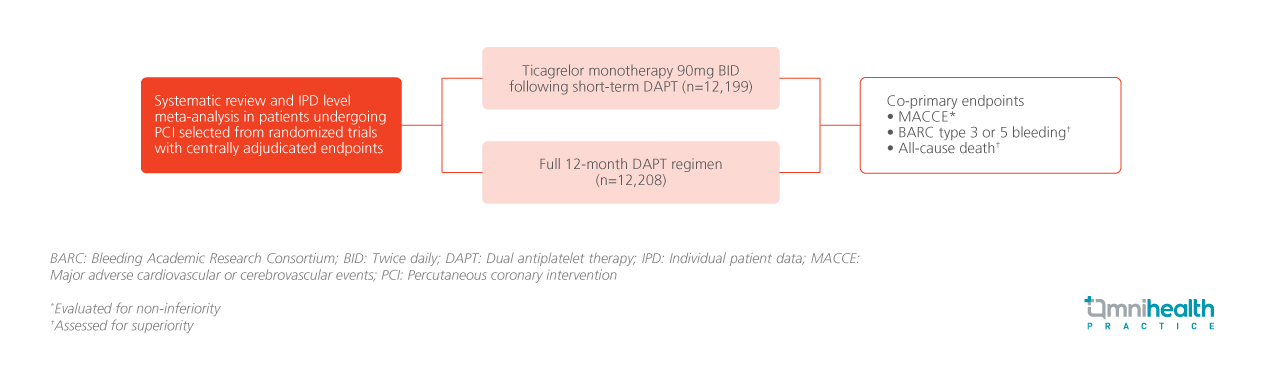
STUDY DESIGN
The 2023 ESC guidelines advocate for a standard treatment of 12-month dual antiplatelet therapy (DAPT) in patients with acute coronary syndrome (ACS) as a Class I recommendation with a high level of evidence.1 For those who may benefit from shorter DAPT regimens depending on their specific high bleeding risk factors, a shorter course of less than 12 months may also be recommended.1 Furthermore, the selection of single antiplatelet therapy following the cessation of DAPT has been recognized as an important factor that may influence outcomes when comparing abbreviated vs. standard DAPT regimens.1
A systematic review and individual patient data (IPD) level meta-analysis of randomized trials with centrally adjudicated endpoints were conducted to assess the efficacy and safety of ticagrelor monotherapy at 90mg twice daily following short-term dual antiplatelet therapy (DAPT) compared to a full 12-month DAPT regimen in patients undergoing percutaneous coronary intervention (PCI).1 Studies that were randomized before the common DAPT phase were censored.1 The final review included the GLASSY, SMART-CHOICE, TICO, TWILIGHT, T-PASS, ULTIMATE-DAPT studies.1
The presentation reported three ranked co-primary endpoints.1 The first was major adverse cardiovascular or cerebrovascular events (MACCE), a composite of all-cause death, myocardial infarction (MI), or stroke evaluated for non-inferiority.1 The second and third co-primary endpoints were Bleeding Academic Research Consortium (BARC) type 3 or 5 bleeding and all-cause death, both of which were assessed for superiority in the intention-to-treat (ITT) population.1 Additionally, trial sequential analysis was performed on the three co-primary endpoints.1
FINDINGS
|
Co-primary endpoints: |
|||||
|
|||||
|
|||||
|
|||||
|
"We provide conclusive evidence that DAPT de-escalation to ticagrelor monotherapy preserves the fatal and non-fatal ischemia and halves major bleeding risks compared with 1-year DAPT "
Professor Marco Valgimigli
Cardiocentro Ticino Institute
Lugano, Switzerland
- Valgimigli M, et al. Ticagrelor monotherapy or DAPT after drug-eluting coronary stenting in patients with or without acute coronary syndrome. A patient-level meta-analysis of randomized controlled trials. Presented at the European Society of Cardiology Congress 2024; Aug 30-Sep 2, 2024.
Prolonged use of DAPT with ticagrelor in a high-risk post-myocardial infarction patient: A local case report
After myocardial infarction (MI) and/or percutaneous coronary intervention (PCI), dual anti-platelet therapy (DAPT) with aspirin and platelet adenosine diphosphate P2Y12 receptor inhibitors, such as clopidogrel, prasugrel or ticagrelor,

