CONFERENCE UPDATE: ACC
Ticagrelor monotherapy after 1 month of DAPT outperforms 12-month DAPT post-PCI in reducing bleeding events in ACS patients: The ULTIMATE-DAPT trial
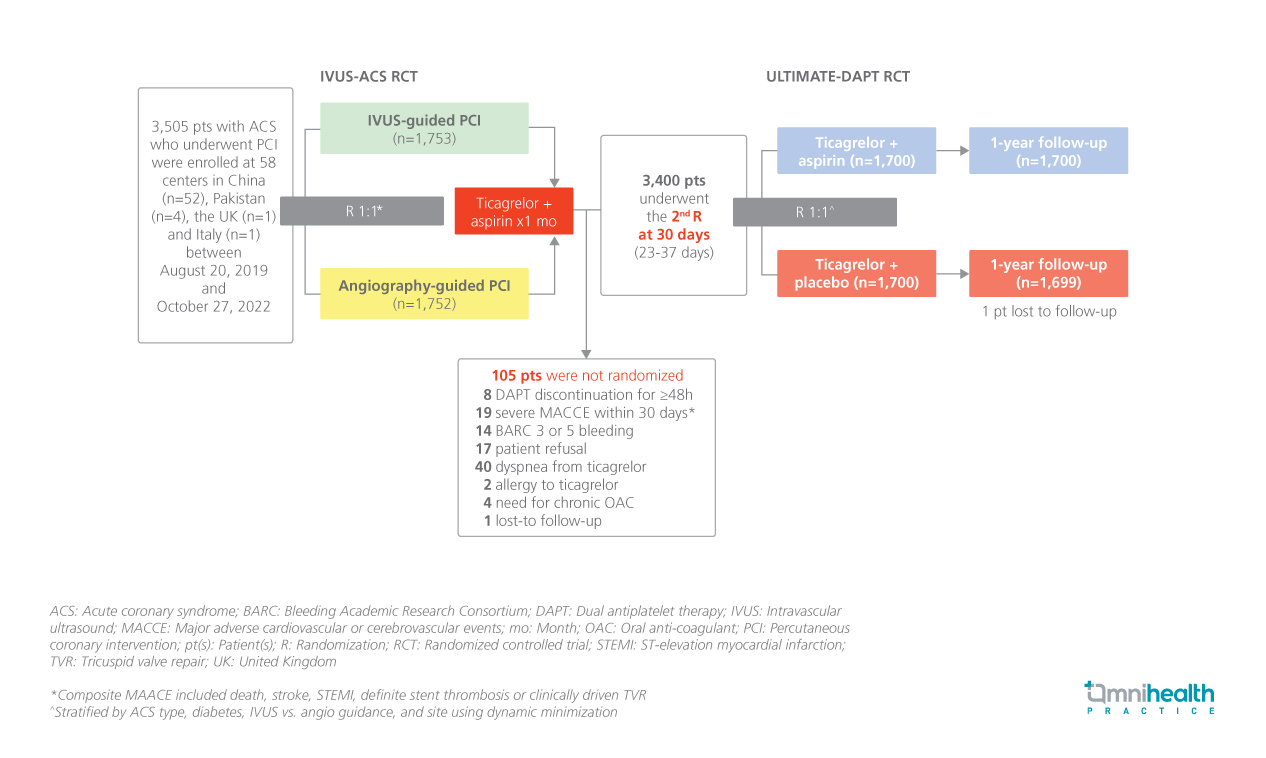
STUDY DESIGN
Dual antiplatelet therapy (DAPT) with aspirin + a potent P2Y12 receptor inhibitor for 12 months is the mainstay for patients presenting with acute coronary syndrome (ACS) who have been treated with percutaneous coronary intervention (PCI) to prevent myocardial infarction (MI) and stent thrombosis.1 However, limited data are available on the use of a single antiplatelet therapy (SAPT) with a potent P2Y12 inhibitor starting 1 month after PCI in ACS, and no placebo-controlled trials have been performed.1
ULTIMATE-DAPT is a large-scale, international, multicenter, placebo-controlled, double-blind randomized trial aimed to determine whether ticagrelor alone compared with ticagrelor + aspirin at 30 days after PCI in patients with ACS could reduce clinically relevant bleeding without an accompanying increase in major adverse cardiovascular or cerebrovascular events (MACCE).1
The study included 3,400 patients who presented with ACS within 30 days before randomization, with either biomarker-positive non-ST-elevation myocardial infarction (NSTEMI) or ST-elevation myocardial infarction (STEMI), or biomarker-negative unstable angina (diameter stenosis ≥90%, ruptured plaque, or thrombotic lesion), had undergone intravascular ultrasound (IVUS)- guided or angiography-guided PCI, and remained event-free after PCI with contemporary drug-eluting stents (DES) for 1 month on ticagrelor + aspirin.1 Patients were randomized 1:1 to receive ticagrelor + aspirin (n=1,700) or ticagrelor + placebo (n=1,700).1
The primary effectiveness endpoint was clinically relevant bleeding (Bleeding Academic Research Consortium [BARC] types 2, 3 or 5) between 1 and 12 months post-PCI, powered for superiority testing.1 The primary safety endpoint was a composite MACCE (cardiac death, MI, ischemic stroke, definite stent thrombosis, or clinically driven tricuspid valve repair) between 1 and 12 months post-PCI, powered for non-inferiority testing.1
FINDINGS
|
Efficacy endpoint: |
|
|
|
|
|
|
Safety: |
|
|
|
|
These results, in concert with prior trials, warrant updating the guidelines and change in practice to treat most patients with ACS after PCI with 1-month DAPT only followed by conversion to SAPT with a potent P2Y12 inhibitor
Dr. Gregg W. Stone
Icahn School of Medicine at Mount Sinai,
New York, United States

