CONFERENCE UPDATE: EULAR 2024
Attaining LLDAS and reducing GC use with anifrolumab in SLE patients: The TULIP-LTE study
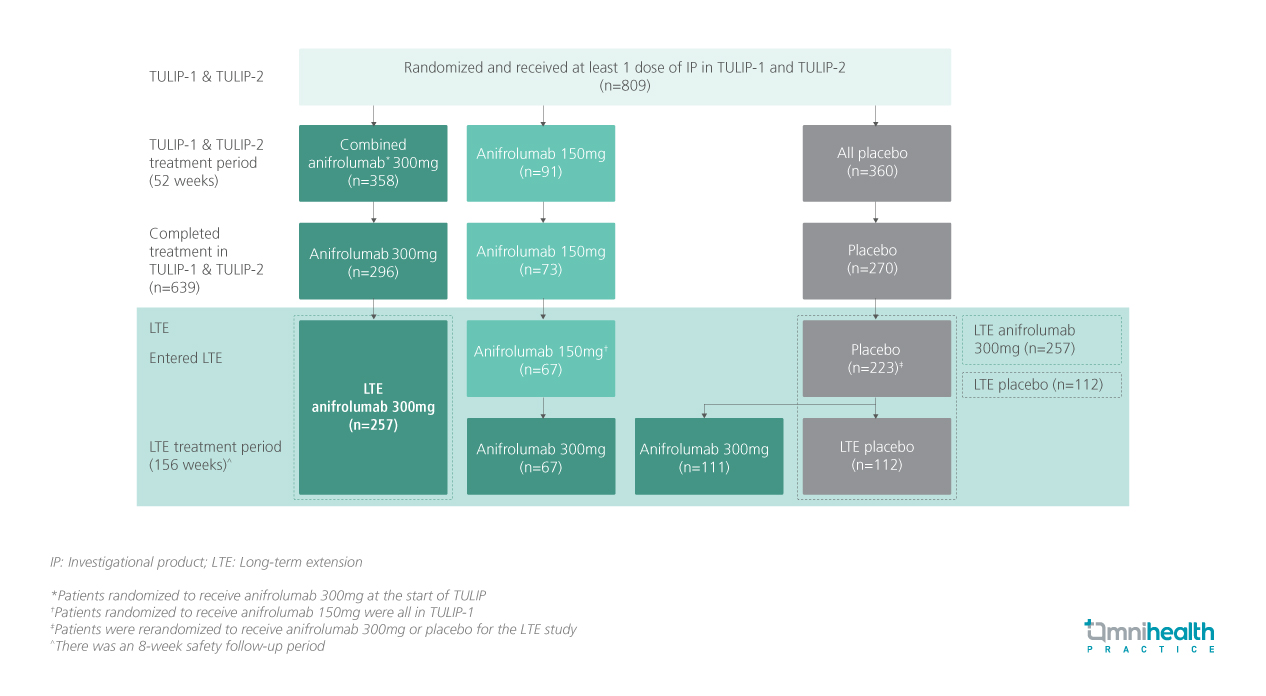
STUDY DESIGN
In patients with systemic lupus erythematosus (SLE), Lupus Low Disease Activity State (LLDAS) attainment is associated with reduced flares, oral glucocorticoid (GC) dosing, damage accrual, and mortality.1 The post-hoc analysis of the 52-week phase 3 TULIP-1/-2 trials showed that patients with SLE who received anifrolumab were more likely to achieve LLDAS over the placebo group.1
TULIP-LTE is the extension of the TULIP-1/-2 trials and is a 3-year, randomized, blinded, placebo-controlled study that evaluated the long-term impact of anifrolumab on LLDAS attainment and GC dose reduction to ≤5mg per day in patients with moderate to severe SLE.1 This study included patients who had received intravenous (IV) anifrolumab 300mg (n=257) or placebo (n=112) every 4 weeks (Q4W) along with the standard therapy from TULIP-1/-2 through week 208.1 Patients were followed from baseline (of TULIP-1/-2) through the end of the LTE (week 208).1
The definition of response in this study was LLDAS attainment and GC dose reduction to ≤5mg per day at the same visit (LLDAS+GC≤5).1 LLDAS attainment was defined as a composite of SLE Disease Activity Index 2000 (SLEDAI-2K) score of ≤4 without major organ activity, no new disease activity, Physician’s Global Assessment (PGA) score of ≤1, prednisone or equivalent ≤7.5mg per day, standard immunosuppressant dosing, no use of restricted medications (over the TULIP-1/-2 period only), and no investigational product (IP) discontinuation.1 Patients who discontinued IP or withdrew from the study were considered non-responders from that visit onwards.1 LLDAS+GC≤5 analyses also included the cumulative time and sustained times spent in LLDAS+GC≤5.1 Results were stratified by the timing of disease onset into established disease (SLE diagnosis >2 years before randomization) or recent disease (SLE diagnosis ≤2 years before randomization).1
FINDINGS
|
Primary outcome: |
|
|
|
|
|
| Secondary outcomes: |
|
|
|
“These benefits with anifrolumab over placebo were observed in the overall population and patients with established disease”
Dr. Eric Morand
Monash University
Melbourne, Australia

