RESEARCH SPOTLIGHT
Exploring the association of systemic glucocorticoids and incident major adverse cardiovascular events in patients with rheumatoid arthritis based on real-world data
In brief
Despite their exceptional anti-inflammatory properties, the use of systemic glucocorticoids (GCs) for the treatment of rheumatoid arthritis (RA) remains controversial due to the potential side effects, particularly their role in elevating patients’ risk of cardiovascular (CV) events.1 A population-based cohort study was conducted in Hong Kong to assess the impact of systemic GC dose and duration on the CV risk, specifically the incidence of major adverse CV events (MACEs), in patients with RA.1 With a mean follow-up duration of 8.7 years, 7.0% (n=860) of the study population (n=12,233) experienced MACEs.1 Patients who had received a daily prednisolone dose of ≥5mg had an approximately 2-fold increased risk of developing MACEs compared to those with no GC use [erythrocyte sedimentation rate (ESR) model: HR=2.02; 95% CI:1.72-2.37; p<0.001; C-reactive protein (CRP) model: HR=1.87; 95% CI:1.60-2.18; p<0.001].1 In contrast, a daily prednisolone dose of <5mg did not appear to be associated with elevated MACE risk.1
Background
Previous clinical studies have established that the application of systemic GCs can be detrimental to patients’ blood pressure, glycemic metabolism, and lipid profile, manifesting an elevated risk of CV events.1 As such, RA management guidelines from the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR) only advocated for short-term (≤3 months) usage of systemic GCs.1 Nevertheless, chronic usage of systemic GCs is reported in roughly 60% of patients with RA, mainly for alleviating synovitis symptoms.1
Considering therapeutic properties and toxicities of GCs, further investigation is required to better elucidate the association between systemic GCs and CV risk, controlling the effect of inflammation, traditional CV risk factors and other co-medications.1 In particular, the implications of systemic GC dosage or duration toward patients’ long-term CV risk remain controversial.1 Hence, researchers from The Chinese University of Hong Kong’s Faculty of Medicine conducted a retrospective, longitudinal, population-based study to investigate the effects of systemic GC dose and duration on the risk of MACEs in patients with RA.1
Methodology
In this retrospective study, clinical data of 12,233 adult RA patients who attended public hospital services from January 2006 to December 2015 were collected from the Hospital Authority Data Collaboration Laboratory database.1 Patients with MACE that preceded their diagnosis of RA were excluded from this study.1 All patients were followed up until December 2018.1 The primary outcome of this study was the first incidence of MACE, which included myocardial infarction (MI), unstable angina, ischemic or hemorrhagic cerebrovascular accident (CVA), transient ischemic attack, and CV death.1 Time-varying univariate and multivariable analyses were conducted to analyze the association between various covariates (including patient characteristics, traditional CV risk factors, inflammatory markers, and medications) and the incidence of MACE. Propensity score-matched sensitivity analyses were also performed.1
The study population consisted of RA patients who were mostly female (81.4%), had a mean age of 57.7 years, and a mean disease duration of 0.7 years, with 37.7% of patients receiving systemic GC treatment at baseline.1 In addition, 2.8%, 55.6% and 76.7% of patients were prescribed biological disease-modifying antirheumatic drugs (bDMARDs), methotrexate (MTX), which is a conventional synthetic DMARD (csDMARD), and non-steroidal anti-inflammatory drugs (NSAIDs) respectively.1 In terms of comorbidities, 34.5% of patients were diagnosed with hypertension (HT), while 7.9% and 3.4% of patients were diagnosed with hyperlipidemia (HL) and diabetes mellitus (DM) respectively.1
Results
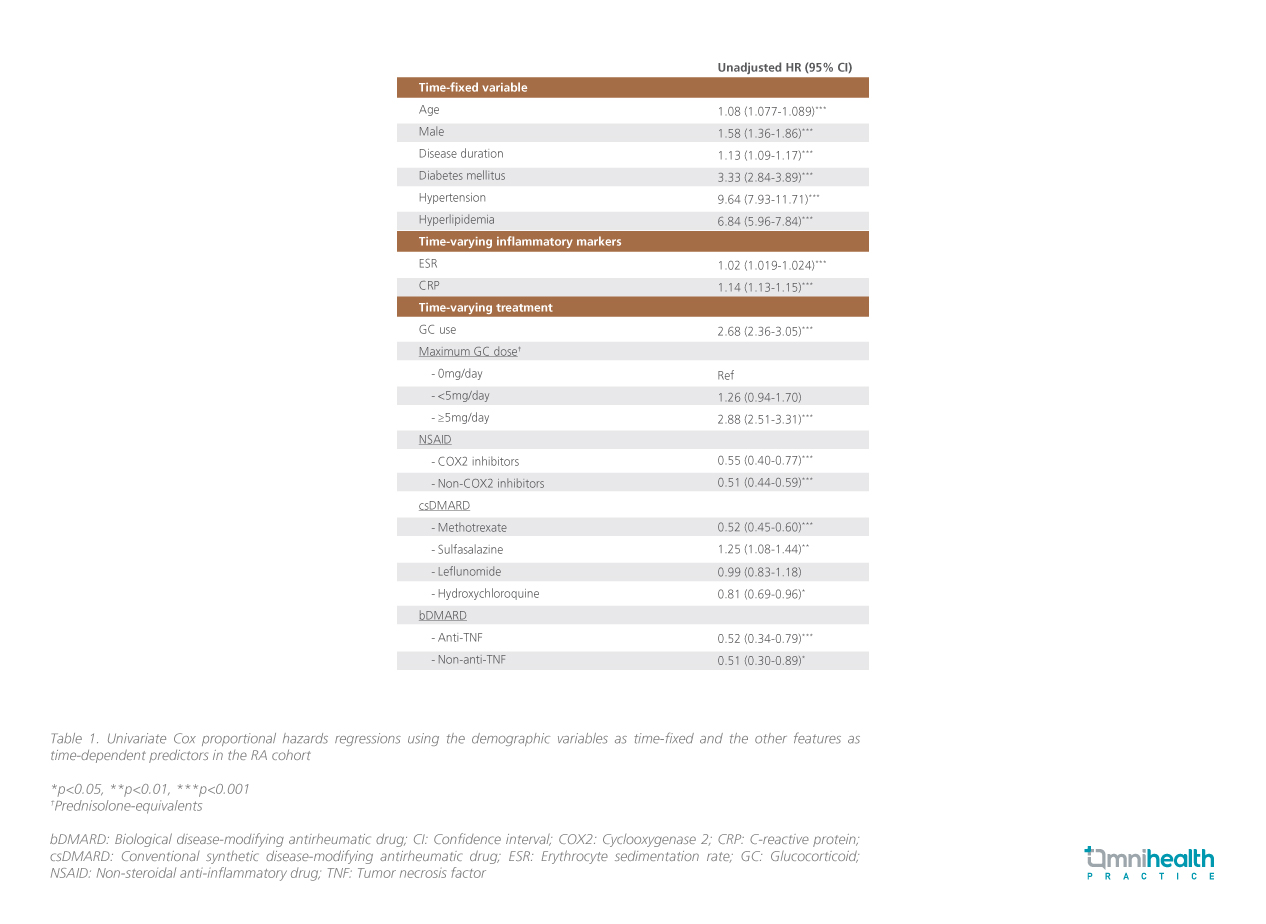
In general, 7% of patients developed a MACE throughout the study period, leading to a crude incidence rate of 8.13 per 1,000 person-years.1 Results from the univariate analysis (table 1) indicated that patients with conventional CV risk factors (older age, male gender, having DM, HT, and HL) and inflammatory burden (having longer disease duration, higher ESR and CRP levels) had a significantly higher risk of developing MACEs (p<0.001 for all).1 Treatment-wise, patients who received systemic GC treatment had a 2.7-fold increased risk of developing MACEs when compared to those without systemic GC treatment (HR=2.68; 95% CI: 2.36-3.05; p<0.001).1 In particular, daily prednisolone of ≥5mg was associated with almost a 3-fold increase in patients’ risk of incident MACEs (HR=2.88; 95% CI: 2.51-3.31; p<0.05), while no significant association was observed between a daily prednisolone dose of <5mg and incident MACEs (HR=1.26; 95% CI: 0.94-1.70; p=0.12).1 In contrast, patients who received other antirheumatic medications (NSAID, MTX, bDMARDs) were associated with a reduced risk of MACEs (p<0.05 for all).1
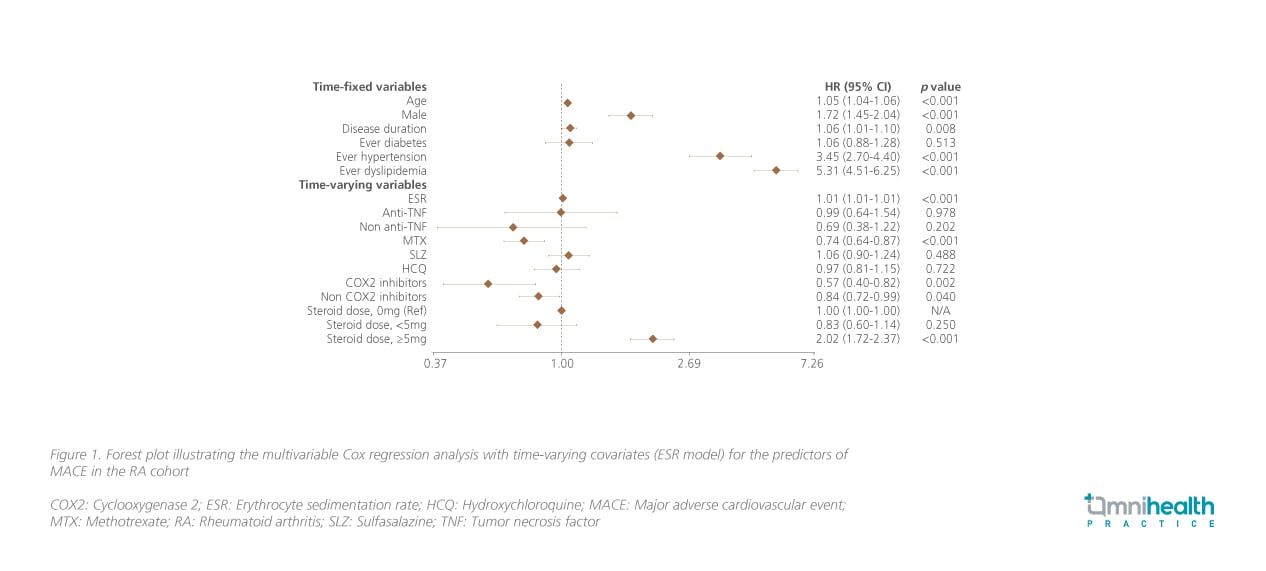
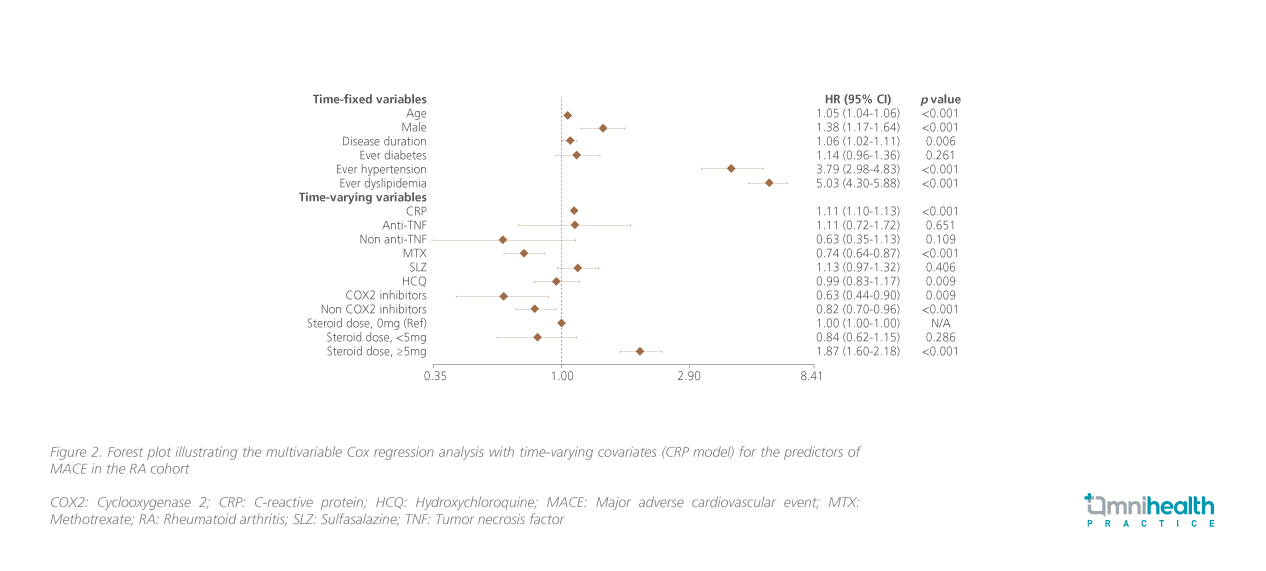
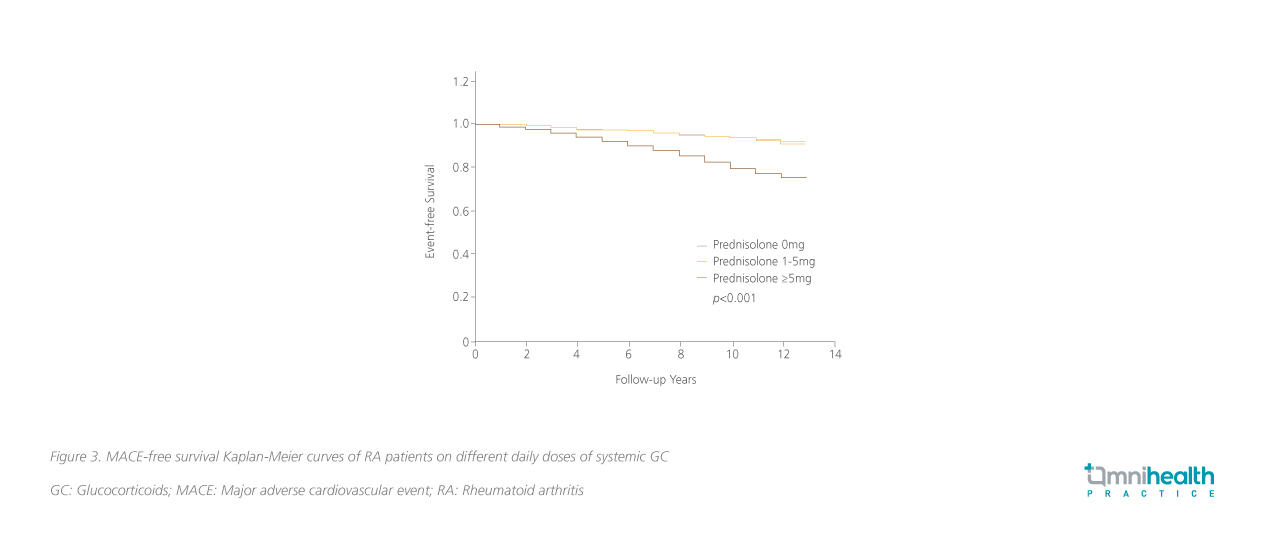
Similar trends in the relationship between systemic GC use and incident MACEs can be seen in the multivariable analysis (figure 1 & 2).1 Compared to patients with no systemic GC usage, significantly higher risk of MACE was still observable among patients who received prednisolone ≥5mg daily (ESR model: HR=2.02; 95% CI: 1.72-2.37; p<0.001; CRP model: HR=1.87; 95% CI: 1.60-2.18; p<0.001) and patients who received prednisolone <5mg daily were not found to have increased risk of developing MACEs (ESR model: HR=0.83; 95% CI: 0.60-1.14; p=0.25; CRP model: HR=0.84; 95% CI: 0.62-1.15; p=0.286).1 The risk of MACE among patients who received prednisolone ≥5mg daily was elevated by 7% per month. These patients had significantly reduced MACE-free survival compared to patients with daily prednisolone <5mg and 0mg (p<0.001) (figure 3).1
Discussion
During an interview with Omnihealth Practice, Dr. So, Ho, one of the joint first authors of this study, explained the reasoning behind the application of GC in treating RA patients in current clinical practice. “Despite their possible side effects, we acknowledge GC’s remarkable and immediate clinical effectiveness in controlling RA disease activity. Hence, GC treatments should be restricted to the shortest duration at the lowest possible dose taking into consideration the patients’ CV risk profile,” Dr. So commented.
Aside from limiting the dose and duration of GC use, Dr. So emphasized the importance of a comprehensive management plan in controlling CV risks in RA patients. Dr. So remarked, “Conventional CV risk factors such as HT and HL remain dominant in contributing towards the overall CV risk of RA patients. Uncontrolled inflammation is also a crucial predictor of MACE. As such, regular CV risk assessment, adequate risk factor management, and tight RA disease activity control with effective therapeutic agents should be implemented in all patients with RA.” Dr. So shared a clinical case of a 65-year-old male patient with RA on long-term GC treatment who developed MACEs.
Case sharing
A 65-year-old male ex-smoker with good past health was diagnosed with seropositive RA in 2005. GC was prescribed in combination with csDMARDs to the patient at diagnosis. Despite multiple csDMARDs, his RA disease control was suboptimal. The patient continued his GC treatment (prednisolone 5mg daily) until 2015 when he developed a MACE. The patient complained of chest pain for 3 days and was diagnosed to have ST-elevation MI with delayed presentation. Cardiac magnetic resonance imaging (MRI) showed a total occlusion of the mid-left anterior descending artery (LAD) with transmural infarction and satisfactory cardiac function. Medical treatment was adopted.
After the patient’s cardiac condition stabilized, bDMARD was started. In addition, the patient was advised to refrain from smoking. Ever since the initiation of bDMARD, improvements in RA disease control have been observed and GC was gradually weaned off. Thus far, the patient’s condition remained stable.
Conclusion
In conclusion, this study identified an increased risk of incident MACEs among patients with RA who received systemic GC ≥5mg prednisolone daily based on local real-world clinical data.1 Therefore, it is imperative for clinicians to consider the risks and benefits before initiating systemic GC regimen, and limit their usage to short-term duration at the lowest dose possible in managing patients with RA.1 The importance of CV risk screening and control as well as complete abrogation of arthritis-related inflammation cannot be over-emphasized.

