CONFERENCE UPDATE: EULAR 2022
The 2022 updated guidelines for RA
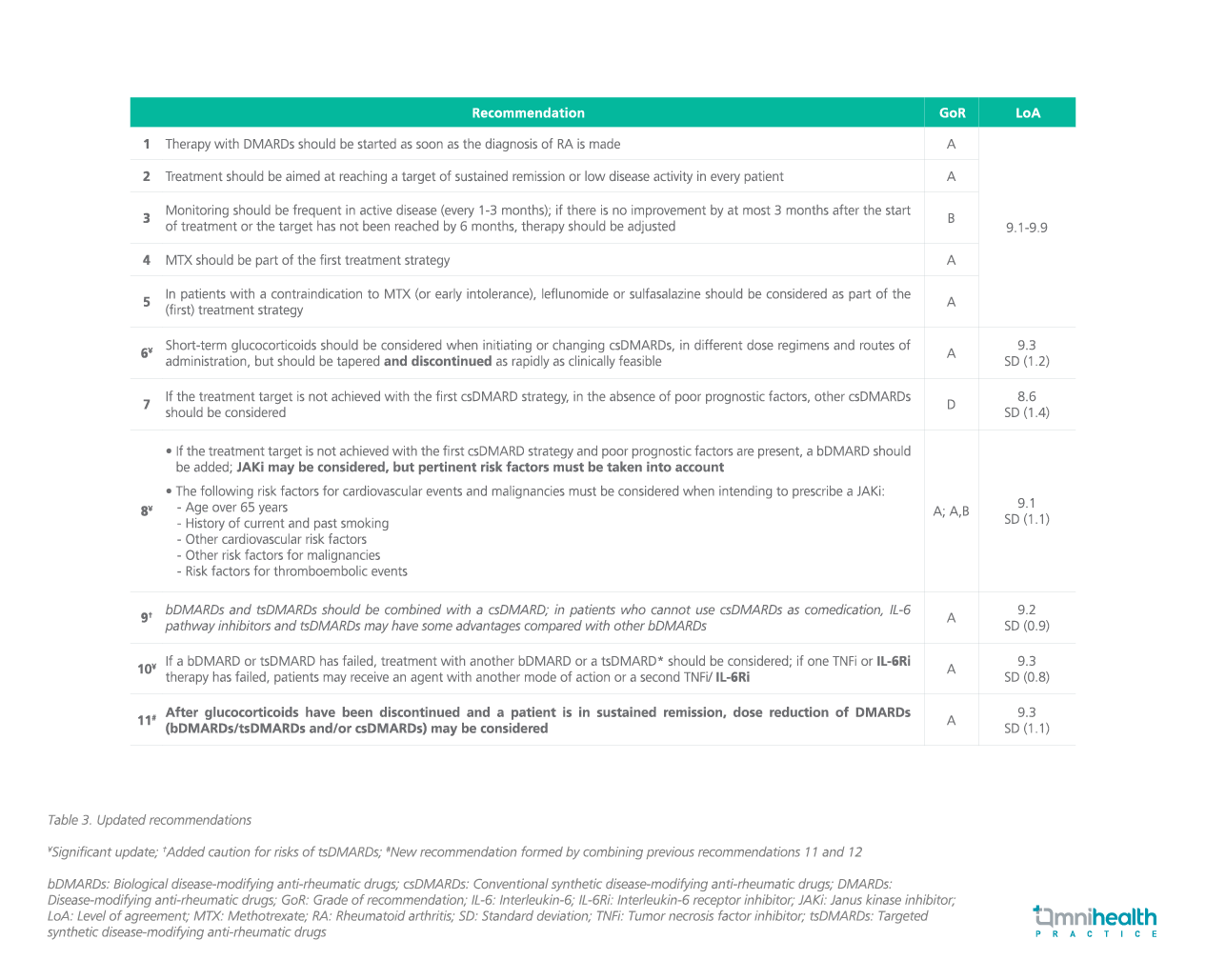
Based on inputs from a global task force on recent systematic literaturereviews (SLRs) regarding rheumatoid arthritis (RA), experts from theEuropean Alliance of Associations for Rheumatology (EULAR) updatedrecommendations for the RA management.1 The previous guidelineswere updated in 2019.2 One main emphasized issue in the update isthe significant swift of glucocorticoid decrease and discontinuationprimarily. This update also includes consideration of using Januskinase inhibitors (JAKis), if the treatment goal is not met with theinitial conventional synthetic disease-modifying anti-rheumatic drugs(csDMARDs) therapy and there are poor prognostic indicators, afterthoroughly reviewing the relevant risk factors. A third importantaddition to the guidelines is the recommendation that if 1 tumornecrosis factor inhibitor (TNFi) or interleukin-6 receptor inhibitor (IL-6Ri) therapy fails, patients may receive an agent with another mode ofaction or a second TNFi or IL-6Ri.1
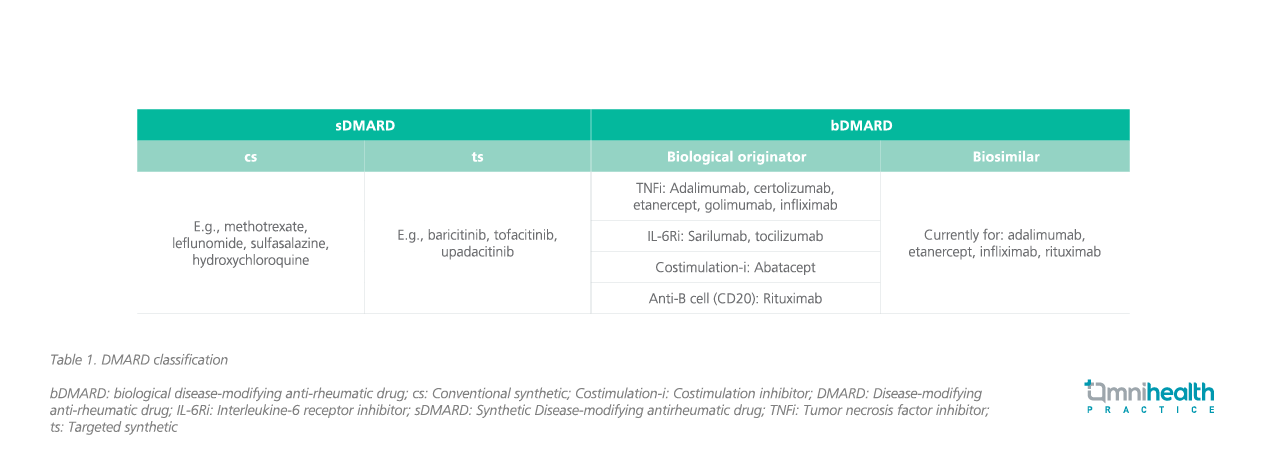
Disease-modifying anti-rheumatic drugs (DMARDs) consist ofsynthetic (s) or biological (b) DMARDs, and as previously stated inpublished data, the DMARD classification used here is presentedin table 1.2 The guidelines have 5 overarching principles wherethe recommendations for the RA management are based on.1These 5 overarching principles remain unchanged from the 2019recommendations and are listed in table 2.1 Each recommendation isassigned a level of evidence (LoE) and grade of recommendation (GoR)assessed from the SLRs in accordance with the Oxford Centre forEvidence-based Medicine.3

LoE
- Level 1a refers to evidence from a meta-analysis of randomizedcontrolled trials (RCTs)
- Level 1b corresponds to at least 1 RCT
- Level 2a means that there was at least 1 controlled study withoutrandomization
- Level 2b means that there was at least 1 type of quasi-experimentalstudy
- Level 3 corresponds to descriptive studies, such as comparative,correlation, or case-control studies
- Level 4 refers to expert committee reports or opinions and/orclinical experience of respected authorities
- Level 5 refers to expert opinion without explicit critical appraisal, orbased on physiology, bench research, or “first principles”
GoR
- Garde A (very high level) means consistent level 1 studies
- Grade B (high level) indicates consistent level 2 or 3 studies, orextrapolations from level 1 studies
- Grade C means level 4 studies, or extrapolations from level 2or 3 studies
- Grade D reflects level 5 evidence or troublingly inconsistent orinconclusive studies of any level
Following the in-person meeting, each recommendation was given theappropriate LoE and strength of recommendation based on the SLRs,as decided by the task force. After inclusion of this information, therecommendations were evaluated for the levels of agreement (LoA)anonymously via email. Each recommendation received an assessmenton a scale of 0-10 with a score of 0 meaning “no agreementwhatsoever” and a score of 10 meaning “full agreement”; the meanvalues and standard deviations (SD) of these votes were presented.1
The 2022 update consists of a total of 11 recommendations: 6 areunchanged from the previous recommendations (#1, 2, 3, 4, 5 and7), 3 are significantly updated (#6, 8, and 10), 1 recommendation hasasterisks for risks of targeted synthetic (ts) DMARDs added (#9), and 1was newly formulated (#11) by combining previous recommendations#11 and #12.1 The updated guidelines are listed in table 3.1