CONFERENCE UPDATE: ESC 2023
Empagliflozin yields clinical benefit in patients hospitalized for acute HF: The EMPULSE trial
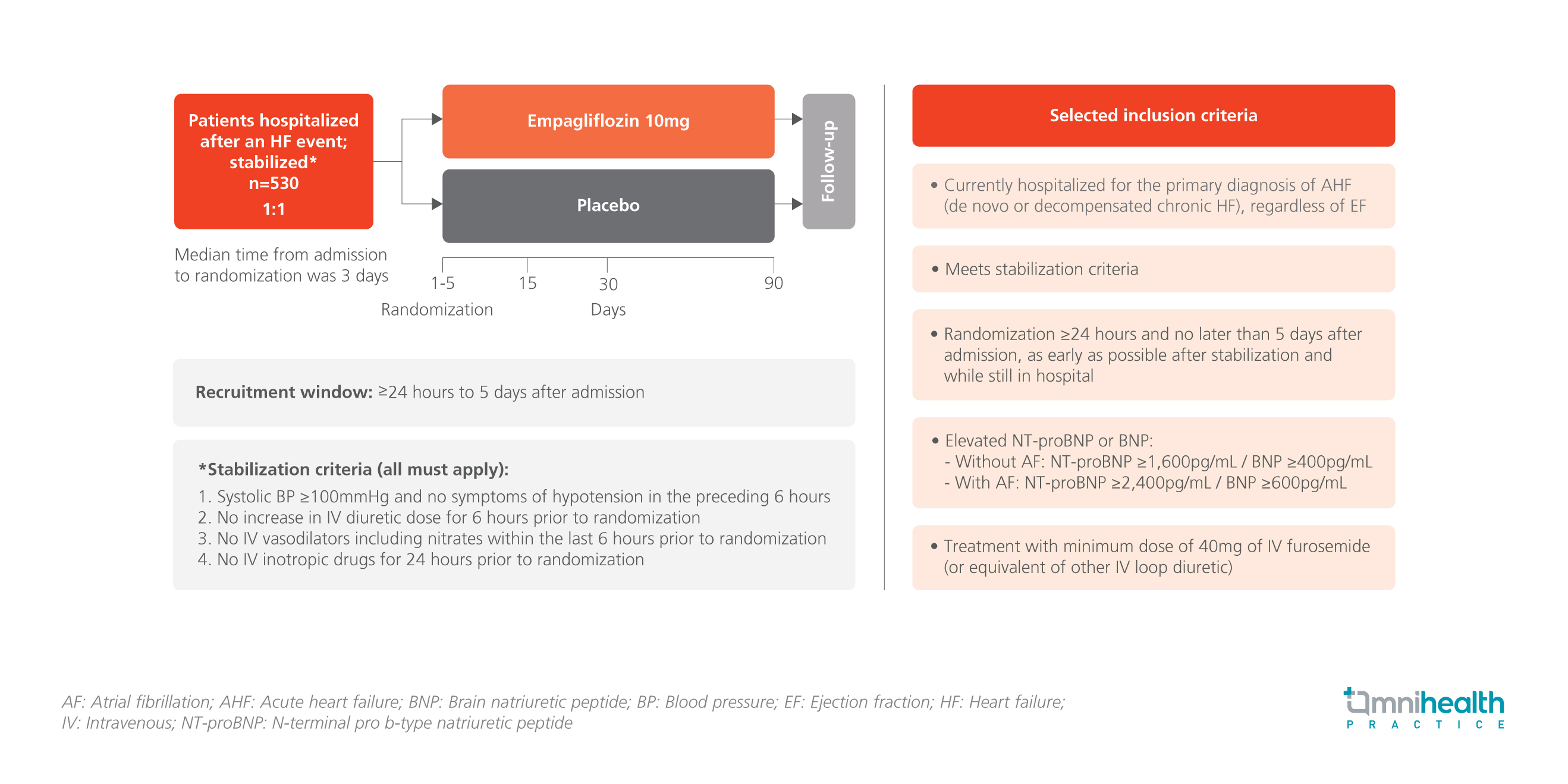
STUDY DESIGN
Sodium-glucose cotransporter 2 (SGLT2) inhibitors have been shown to effectively treat chronic heart failure (HF) in multiple large clinical trials.1 However, the potential clinical benefits of empagliflozin, the SGLT2 inhibitor, in patients hospitalized for acute HF are currently unclear.1 The EMPULSE trial was designed to address this knowledge gap by recruiting 530 patients who had been hospitalized after an acute HF event (HFE).1 The participants were randomized 1:1 to receive 10mg oral empagliflozin (n=265) or placebo (n=265) within 1-5 days of admission.1 Notably, patients were also included regardless of ejection fraction (EF) or diabetes status.1
All patients had met the stabilization criteria of systolic blood pressure (BP) of ≥100 mmHg and without the need for intravenous (IV) vasodilators or inotropic drugs.1 Additionally, they had congestion as evidenced by elevated N-terminal pro-B-type natriuretic peptide (NT-proBNP) or BNP and the need for diuretic therapy but without an increase in the dose 6 hours prior to randomization.1 Patients were followed up for 90 days with assessments conducted on days 15, 30 and 90.1
The primary endpoint of the study was the clinical benefit at 90 days assessed using a win ratio, which is a hierarchical composite of death, number of HFEs [i.e., hospitalizations from HF (HHFs), urgent HF visits and unplanned outpatient visits], time to first HFE, and the change in quality of life (QoL) assessed by the Kansas City Cardiomyopathy Questionnaire Total Symptom Score (KCCQ-TSS).1 The selected secondary endpoints included time to all-cause death, first HFE, KCCQ-TSS change from baseline, and body weight (kg) change from baseline after 90 days of treatment.1
FINDINGS
| Primary endpoint: |
|
|
|
| Secondary endpoints: |
|
|
|
|
|
| Safety: |
|
|
|
"All these results concluded early initiation of empagliflozin vs. placebo in patients hospitalized for acute HF is good and beneficial for the patients and should be done."
Professor Christiane E. Angermann
Comprehensive Heart Failure Centre,
University of Würzburg,
Germany

