CONFERENCE UPDATES: ASCO 2023
Abemaciclib + ET shows consistent treatment benefits for high-risk EBC patients across all age groups: Subgroup analysis of the monarchE trial
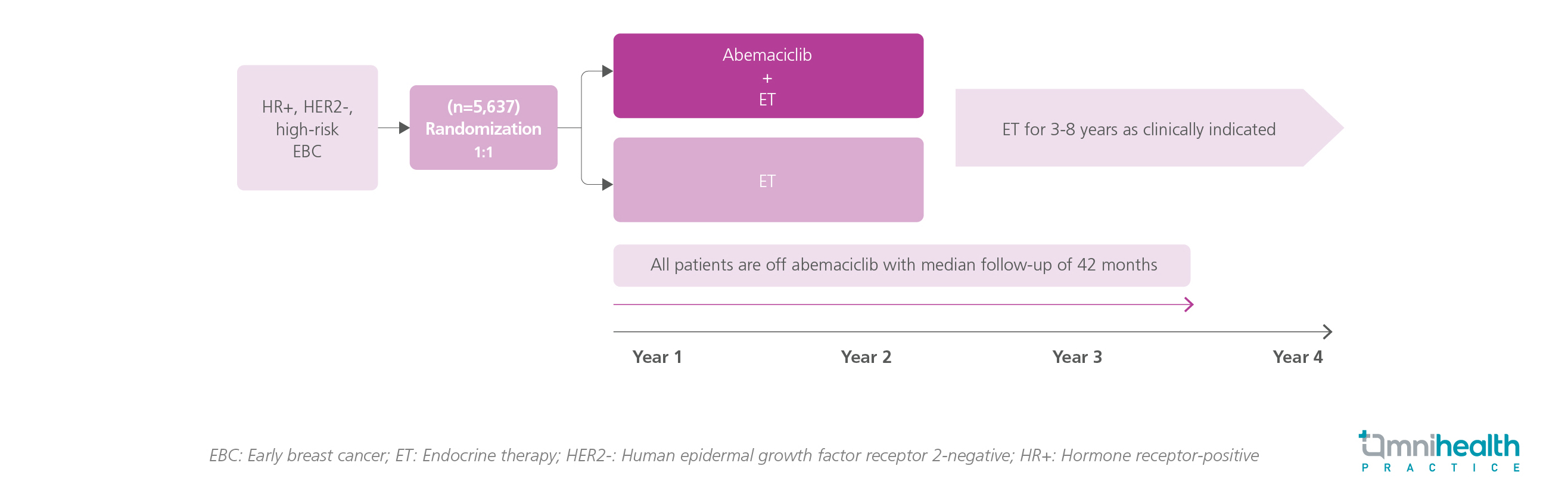
STUDY DESIGN
The monarchE trial was the only adjuvant trial that assessed the benefits of adding 2 years of adjuvant abemaciclib to endocrine therapy (ET) in high-risk hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-), early breast cancer (EBC) patients.1 This trial randomized 5,637 patients in 1:1 into the abemaciclib + ET group or the ET alone group.1 In the primary analysis, 2-year abemaciclib demonstrated significant risk reductions in invasive disease-free survival (IDFS) (HR=0.664; 95% CI: 0.578-0.762) and distant relapse-free survival (DRFS) (HR=0.659; 95% CI: 0.567-0.767) in the overall intention-to-treat (ITT) population.1 In a subsequent subgroup analysis, the dataset was divided into 2 age subgroups, i.e., patients aged <65 years (n=4,787) and ≥65 years (n=850).1 The median follow-up of monarchE was 42 months.1
The findings showed that abemaciclib in combination with ET resulted in a consistent treatment benefit across all age subgroups in terms of the IDFS and DRFS risk reduction, despite the higher prevalence of comorbidities and dose modifications in elderly patients aged ≥65 years.1 The IDFS benefit achieved with abemaciclib was maintained at the consistent 4-year IDFS and DRFS rates across all age subgroups.1 Furthermore, quality of life (QoL), measured by the Functional Assessment of Cancer Therapy-Breast (FACT-B) total score, was similar between the 2 arms and the age subgroups.1 The safety profile of abemaciclib was manageable with higher rates of adverse events (AEs) and dose modifications occurring in older patients, particularly those aged ≥75 years.1
FINDINGS
|
Key secondary endpoints: |
|
|
|
|
|
|
|
Secondary endpoint: |
|
|
|
| Safety: |
|
|
"These data supported the use of adjuvant abemaciclib across the age groups and could be used to counsel patients about their expectations of treatment experience."
Dr. Erika Hamilton
Sarah Cannon Research Institute,
Tennessee Oncology,
Nashville, Tennessee, United States

