EXPERT INSIGHT
Overcoming the clinical challenges of breast cancer management: From identifying prognostic factors to achieving personalized treatment
Breast cancer is the most common cancer among females in Hong Kong and accounted for 27.2% and 12.4% of all cancer incidences and deaths in 2018, respectively.1 Over the past decade, the incidence and death rate of breast cancer continued to increase while the challenges of managing the disease remain.1 In a recent interview with Omnihealth Practice, Dr. Kwok, Chi-Hei Carol, specialist in Clinical Oncology, shared her insights on the current clinical challenges of managing breast cancer and the utilization of prognostic factors to better optimize breast cancer treatment.
The heterogeneity of breast cancer necessitates the implementation of personalized treatment
Breast cancer is a heterogeneous disease with prognosis characterized by varied pathological features, distinct response to therapeutics and substantial differences in long-term survival.2 Clinically, patient factors including patient’s age, menopausal status and general health, most importantly, tumor characteristics, are important factors to consider in the management of breast cancer patients.3 Morphologically, the heterogeneity of breast cancer is characterized by the distinct histopathological classifications of the disease.4 Where 80% of invasive breast cancers are classified as invasive ductal carcinoma, 10% of them are invasive lobular carcinoma with the rest classified into less common subtypes, all with different prognosis based on their respective histopathological subtype.4 Moreover, breast cancer can be further subdivided into various molecular subtypes according to the genetic expression of the estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2).4 With improved knowledge in the complex phenotypic and genotypic heterogeneity of breast cancer, true personalized treatment that aims to target every identifiable and actionable factor becomes increasingly needed to implement.
The distinct clinical challenges between early and advanced breast cancer treatment
Due to its heterogeneous nature, early breast cancer (EBC) is often treated based on the pathological and molecular characterization of the tumor.5 Compared with advanced breast cancer (ABC), EBC is potentially curable with some patients even capable of achieving long-term survival with surgery alone.6 As such, the most important treatment goal for EBC is to reduce disease recurrence and improve long-term survival.
In practice, however, many EBC patients have disease recurrence even after receiving adjuvant therapy with unknown recurrence mechanism. Moreover, there is a lack of concrete guidance or biomarkers to guide treatment escalation or de-escalation based on the identified pathological features. Whereas the St. Gallen International Consensus Guidelines have been adopted by local practice, the guidelines as well as the global perspectives on the management of EBC have been evolving rapidly in the recent years.5 While earlier treatment strategy was based on tumor stage only, recent guidelines have included ER, PR, HER2 and other gene expressions as the assessment factors for optimizing EBC treatment in the adjuvant setting.5 Although additional risk factors are now considered to address the heterogeneity of EBC, personalized EBC treatment remains challenging as the recurrence mechanism and concrete guidance for treatment are still undefined.
ABC comprises both inoperable locally advanced breast cancer (LABC) and metastatic breast cancer (MBC). The treatment goal of ABC shifts from the prevention of recurrence towards the improvement of treatment response with palliation of symptoms, prolonging progression-free survival (PFS) and overall survival (OS), at the same time optimizing patient’s quality-of-life. For patients with hormone receptor (HR)+/HER2- ABC, the National Comprehensive Cancer Network (NCCN) Guideline recommends aromatase inhibitor plus cyclin-dependent kinase (CDK) 4&6 inhibitors as the preferred first-line therapy.7 In the second-line and subsequent-line setting, fulvestrant plus CDK4&6 inhibitors can be considered if CDK4&6 inhibitors were not previously used.7 To date, several clinical trials have already been conducted to study the use of targeted therapies on ABC patients with acquired (secondary) endocrine resistance.7
Individualizing breast cancer treatment through predictive/prognostic factors
To help overcome the treatment challenges for breast cancer, an accurate prognosis is needed to tailor the right treatment for each patient individually. For ABC patients, tumor loads, the presence of visceral metastasis as well as the site and number of involvements are usually used as the major prognostic factors in practice.
The most common metastatic organs for breast cancer are the bone, lung, brain and liver. In regard to prognosis, whereas patients with bone only disease have better prognosis, those with brain metastasis have the poorest 2-year OS of 32.3%. Despite the demonstrated clinical benefits of CDK4&6 inhibitors across patients regardless of the metastatic sites, a CDK4&6 inhibitor may not be necessary for patients with bone-only disease due to its better prognosis.8 There is lack of specific guideline as to when CDK4&6 can be omitted and individual consideration needs to be judged in different circumstances.
In addition to histological findings, a longer disease-free interval (DFI) is also widely used as a positive clinical prognostic factor for ABC. Compared to patients with a DFI >2 years, those who have disease relapsed <2 years have a greater risk of death within a year after first recurrence (HR=2.43; 95% CI: 1.53-3.86; p<0.01).9 Clinically, those who relapse during the first 2 years of adjuvant endocrine therapy or have disease progression within the first 6 months of first-line endocrine therapy are considered primary endocrine-resistant and have a poorer prognosis than patients who are endocrine sensitive.10 From experience, patients who have de novo resistance may have a better prognosis than recurrent patients that is likely due to their therapy-naïve status or lower resistance to systemic treatment. However, it should be noted that the absence of salvage treatment at first recurrence is a significant adverse prognostic factor for survival from first relapse (HR=2.55; 95% CI: 1.35-4.82, p<0.01), prompting the need of timely treatment towards ABC patients regardless of their underlying clinical risks.9
Whether young age is a known poor prognostic factor for EBC also remains controversial.10 ABC patients who are <40-years-old typically have a significantly more aggressive presentation than the older ABC patients at >60-years old with a higher frequency of being the HER2+ (25.7% vs. 15.3%) or triple negative subtypes (27.4% vs. 14.6%) and having visceral involvement (36.3% vs. 29.8%).11 From clinical experience, however, the higher frequency of HER2+ and triple negative subtypes among younger patients may have coincidentally increased the mortality risk without age being an actual risk factor itself. In fact, younger ABC patients are associated with a longer median OS (38.8 vs. 35.6 months; p<0.0001) and lower risk of all-cause mortality (HR=0.91; 95% CI: 0.83-0.99) in the long term.11 In practice, treatment for younger patients may be escalated to include ovarian function suppression with aromatase inhibitor to manage the more aggressive presentation. That said, this strategy may not be applicable to all younger patients and age remains an underutilized prognostic factor with a lack of in-depth understanding on its interaction with ABC.
The application of CDK4&6 inhibitors in patients with advanced breast cancer
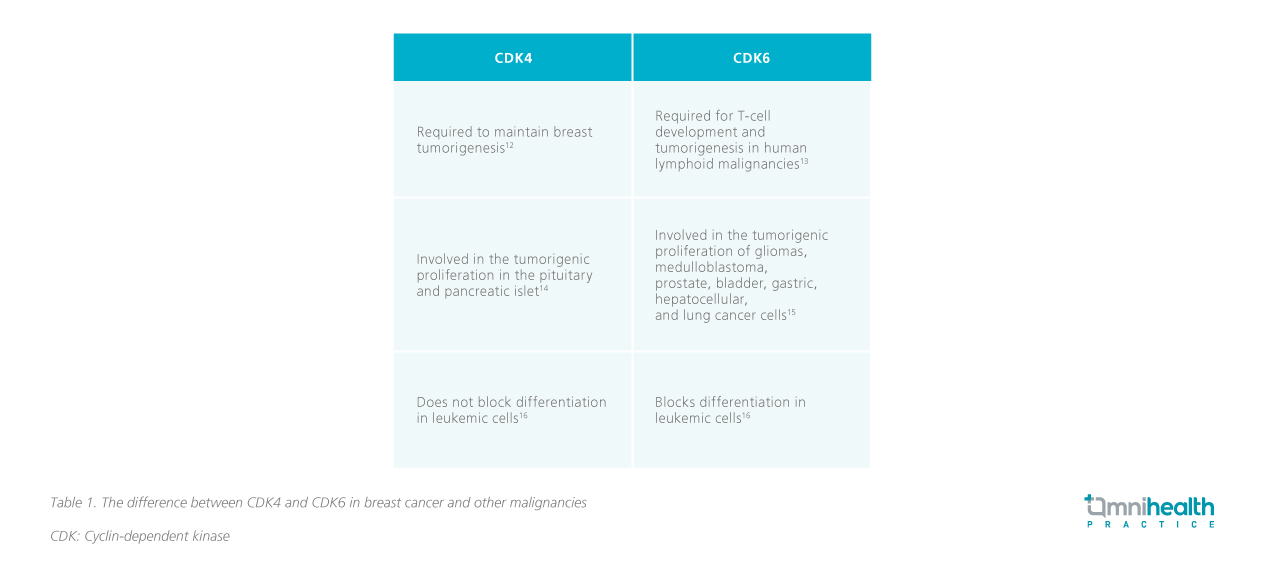
CDK4-associated kinase activity is required to maintain breast tumorigenesis and CDK6 is a critical requirement for T-cell development and tumorigenesis among human lymphoid malignancies (Table 1).12,13 Through a combination approach, CDK4&6 inhibitors can enhance the effectiveness of endocrine therapy and improve the PFS and ORR of ABC patients both as initial therapy and after progression on endocrine therapy, supporting their use across ABC patients regardless of their underlying risk factors.8
Whereas the treatment of ABC is usually tailored to the identified prognostic factors, e.g. tumor load, a subgroup analysis of the MONARCH 2 and 3 studies found that patients with poor prognostic factors including liver metastases, PR-, high tumor grade or short treatment-free interval (TFI) of <36 months consistently derived the largest PFS and ORR benefit from the addition of abemaciclib onto the existing endocrine therapy (Figure 1).8 In principle, CDK4&6 inhibitors are recommended for all HR+/HER2- ABC patients. However, given abemaciclib’s evidence across patients with various poorer prognosis, it could be considered for patients with the need of an escalated treatment.8 Of the three agents, abemaciclib has been evaluated in patients with brain metastases (evidence from phase 2, non-randomized trial only) and may be preferred in this setting. However, this is a very rare occurrence in first- or second-line treatment of hormone receptor-positive breast cancer.

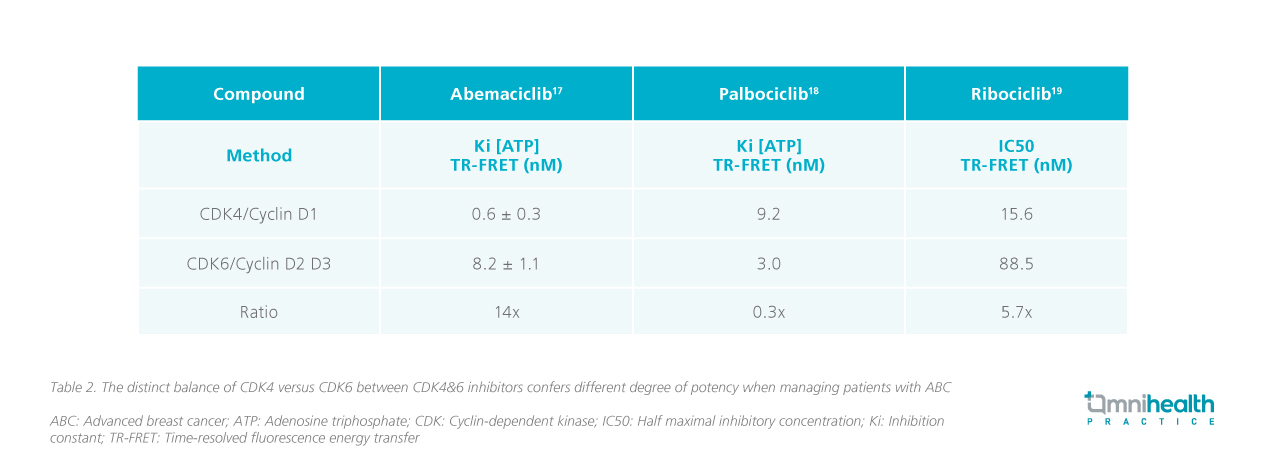
However, while CDK4&6 inhibitors are effective drugs in combination with aromatase inhibitors for the treatment of HR+, HER2- ABC, they are varied in potency with different toxicity profile (Table 2).17-19
 In the MONALEESA-2 and 3 studies, neutropenia of grade 3/4 occurred in 57.1%-62.0% of patients receiving ribociclib vs. 0.8%-1.2% receiving placebo when combined with letrozole or fulvestrant.20,21 In the PALOMA 2 and 3 studies, neutropenia of grade 3/4 occurred in 69.1%-69.6% of patients receiving palbociclib vs. 0%-1.4% in those receiving placebo when similarly combined with letrozole or fulvestrant.22,23 Additionally, only 23.9%-29.9% of patients receiving abemaciclib versus 1.2%-1.7% receiving placebo experienced grade 3/4 neutropenia when combined with fulvestrant or aromatase inhibitor in the MONARCH 2 and 3 studies.24,25 On the other hand, diarrhea was more frequent for abemaciclib but was mainly grade 1 (44.6%).26
In the MONALEESA-2 and 3 studies, neutropenia of grade 3/4 occurred in 57.1%-62.0% of patients receiving ribociclib vs. 0.8%-1.2% receiving placebo when combined with letrozole or fulvestrant.20,21 In the PALOMA 2 and 3 studies, neutropenia of grade 3/4 occurred in 69.1%-69.6% of patients receiving palbociclib vs. 0%-1.4% in those receiving placebo when similarly combined with letrozole or fulvestrant.22,23 Additionally, only 23.9%-29.9% of patients receiving abemaciclib versus 1.2%-1.7% receiving placebo experienced grade 3/4 neutropenia when combined with fulvestrant or aromatase inhibitor in the MONARCH 2 and 3 studies.24,25 On the other hand, diarrhea was more frequent for abemaciclib but was mainly grade 1 (44.6%).26
Based on the MONARCH 3 study and clinical experience, aromatase inhibitors alone can typically provide 14 months or longer PFS and an escalation to CDK4&6 inhibitors may not be necessary for selected patients.25 For example, de-escalating CDK4&6 inhibitors for older patients could potentially reduce the frequency for clinical visits and check-ups. On the other hand, CDK4&6 inhibitors may be insufficient in ABC patients with liver metastasis with impaired liver function, and more aggressive chemotherapy would be required to rapidly control the metastatic disease.
Although CDK4&6 inhibitors have been used in combination with aromatase inhibitors or fulvestrant in both the first- and second-line setting, fulvestrant is typically reserved for the second-line. However, as CDK4&6 inhibitors are commonly used to manage ABC in the first-line setting, and that the re-challenge of CDK4&6 inhibitors after failing first-line therapy is generally not recommended due to the lack of evidence, the combination of CDK4&6 inhibitors with fulvestrant in the second-line setting after failing CDK4&6 inhibitors remains debatable.7
Conclusion
Accumulation of knowledge over time has certainly changed how breast cancer is being managed. Clinicians are shifting away from an “one-size-fits-all” approach to that according to specific molecular aberrations, clinical behaviors and patterns of response to systemic treatments. While personalized treatment is the preferred strategy in managing breast cancer patients, current evidence and understanding on breast cancer biology and its interactions with the immune system and the microenvironment are still insufficient to provide guidance on personalized treatment approach. With treatment guidance now moving towards genetic profiling, a better understanding of the prognostic factors of breast cancer may help clinicians determine the criteria for escalating or de-escalating treatment for a more personalized treatment approach. With the increased understanding on disease mechanisms and molecular characteristics, the optimal treatment sequence for breast cancer patients can be determined and an individualized approach for better tumor control can be achieved in the future.

