CASE REVIEW
A local case sharing: Romosozumab as a novel bone-forming alternative in postmenopausal women with osteoporosis
It is often a challenge to manage serious but asymptomatic illnesses, and osteoporosis is one of them.1,2 Osteoporosis, a metabolic bone disease characterized by low bone density and increased risks of fragility fractures, is common among Hong Kong population aged ≥50 years, with its prevalence measured as high as 37%.3 Nevertheless, the disease remains a seriously underdiagnosed and undertreated medical condition due to its silent nature.1,3 No doubt, increasing screening for osteoporosis to identify the high-risk patients early will certainly help reduce the incidence rates of fractures, but treatment with safe and effective pharmacological agents for fracture reduction is, in fact, equally important. In a recent interview with Omnihealth Practice, Dr. Tse, Yun-Tin Paul, a specialist in Orthopaedics & Traumatology, discussed the treatment principles for osteoporosis and shared a clinical case to demonstrate the effectiveness and safety of romosozumab, a novel bone-forming treatment option, in postmenopausal women with osteoporosis.
Osteoporosis-related fractures are on the rise in Hong Kong
Osteoporosis and its associated bone fractures are imposing tremendous economic and health burdens on the global community.4 This is particularly problematic in Hong Kong for its rapidly growing elderly population and the longest life expectancy in the world.3 Given the high prevalence of osteoporosis and its underdiagnosis in Hong Kong, the Hong Kong Osteoporosis Study (HKOS) was conducted and found that the number of hip fractures caused by osteoporosis has increased by nearly 70% over the past 20 years.5 More importantly, the majority of the osteoporosis patients did not receive pharmacological treatment even after they had experienced bone fractures.6 Therefore, without appropriate intervention, the ageing population is set to result in a considerable increase in the occurrences of fragility fractures, creating a huge burden to our society.3 In these regards, a group of local medical practitioners proposed that in order to mitigate the future fracture risk, all men aged ≥70 years and women aged ≥65 years should receive universal dual-energy X-ray absorptiometry (DEXA) assessment for osteoporosis, and the high-risk groups should be given ≥3 years of anti-osteoporotic treatment.3
Current management approach and challenges for osteoporosis
The main goal of osteoporosis pharmacological treatment is to prevent bone fractures by improving bone strength and reducing the risks of falling and injury.7 The existing osteoporosis treatment options include antiresorptive and bone-forming agents. The former drug class works by decreasing bone resorption and has been proven effective in reducing fracture risks among osteoporotic patients.7 Nevertheless, they do not possess the ability to stimulate bone formation, which is crucial for patients with severe or established osteoporosis.7 Concerning this, the bone-forming agents, which can improve the bone structure and provide a greater fracture risk reduction, are needed.
As of 2019, there was only one bone-forming agent available in Hong Kong.8 Although it has been utilized in clinical practice for more than 10 years in Hong Kong, the daily injection requirement of the older agent was found to have compromised patients’ adherence to the osteoporosis treatment and have led to an increased likelihood of drug discontinuation.9,10 Therefore, a safe and highly effective alternative with a less frequent injection regimen is warranted.
Romosozumab: Dual-acting therapy to provide extra convenience with manageable safety profile
Romosozumab is a humanized monoclonal antibody which inhibits sclerostin, thereby embracing dual actions of increasing bone formation through the activation of bone lining cells as well as preventing bone resorption.12 Due to its high efficacy and favorable safety profile in osteoporotic patients demonstrated in several clinical studies, romosozumab received regulatory approval for osteoporosis treatment in Hong Kong.8 “Previously, we only have one treatment option, now we have an alternative which provides more convenience to patients,” Dr. Tse said. Dr. Tse utilized the novel bone-forming agent, which only needs to be administered once monthly, in his clinical practice and shared a case to demonstrate its effectiveness and safety.
|
Case report |
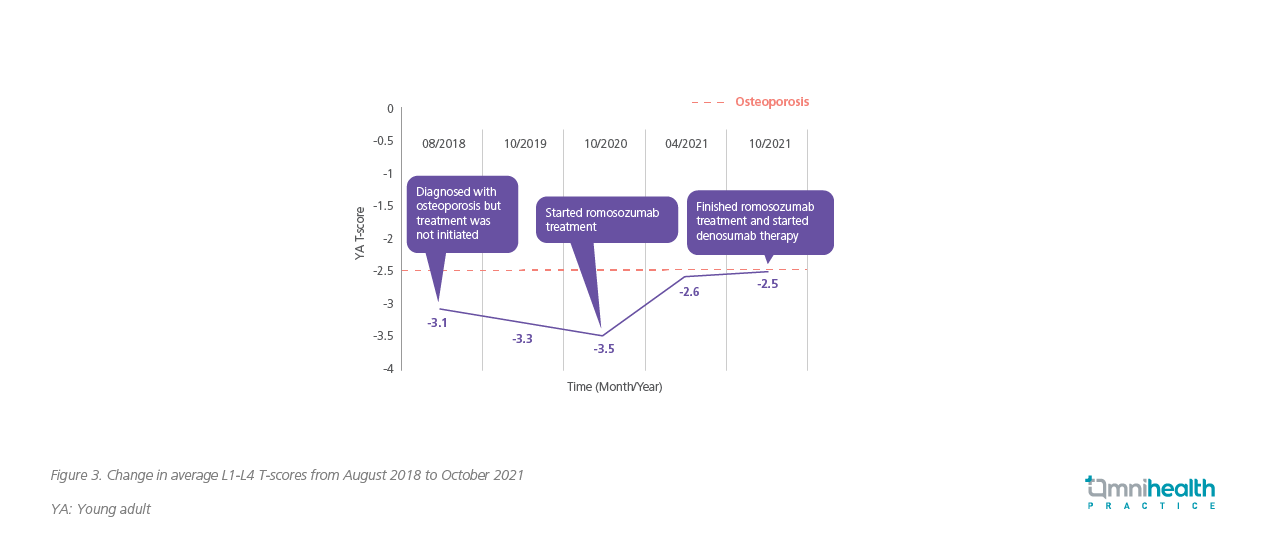
In 2018, a 56-year-old asymptomatic postmenopausal woman was diagnosed with osteoporosis in lumbar spine with an average L1-L4 bone mineral density (BMD) of 3.1 standard deviations below young adult female (i.e. T-score=-3.1). The finding suggested mildly to highly increased risk of minimal trauma fracture. However, anti-osteoporotic treatment was not initiated, and her condition kept deteriorating. The average T-score was reduced to -3.3 a year later. In October 2020, her T-score was further lowered to -3.5, indicating an even higher risk of fracture at the lumbar spine (Figure 1). After discussion with the patient of the potential consequences, she accepted pharmacological treatment. Since the patient preferred a more convenient regimen and did not have any medical history of stroke or other cardiovascular diseases, she started on romosozumab therapy with a monthly subcutaneous injection for 12 months. DEXA scans were performed at month 6 and month 12 to monitor her treatment response.
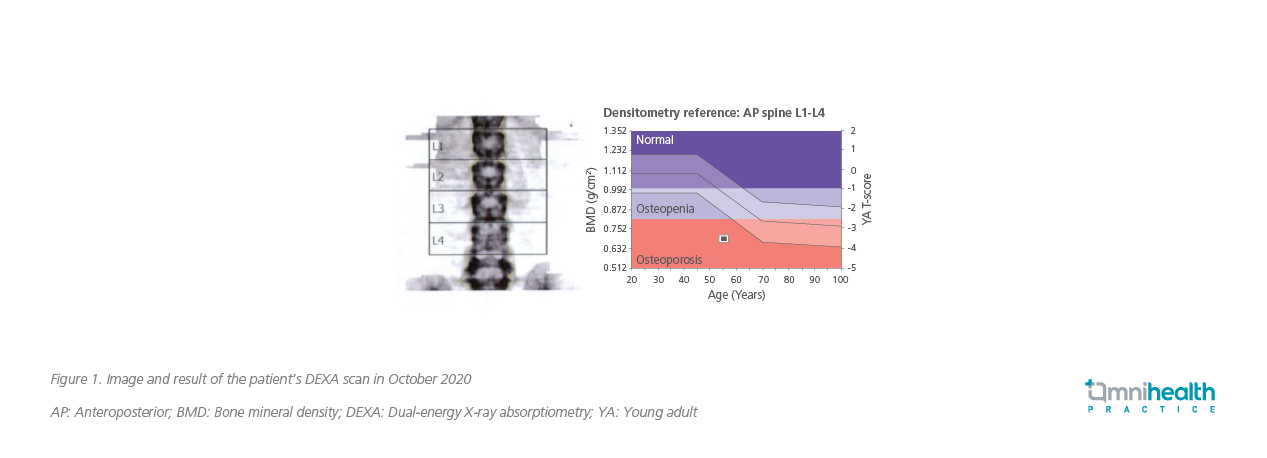
At month 6, the patient’s improvement in BMD of the lumbar spine was remarkable. The DEXA scan showed a significant increase in the average T-score from -3.5 to -2.6 after 6 months of romosozumab treatment. Of note, the improvement was the greatest at L4 where the T-score was increased from -4.1 to -2.9. The response stabilized at month 12 with her average T-score slightly increased from -2.6 to -2.5 (Figure 2). Nevertheless, the improvement in BMD at L2 continued, in which the T-score was increased from -2.6 to -2.1.
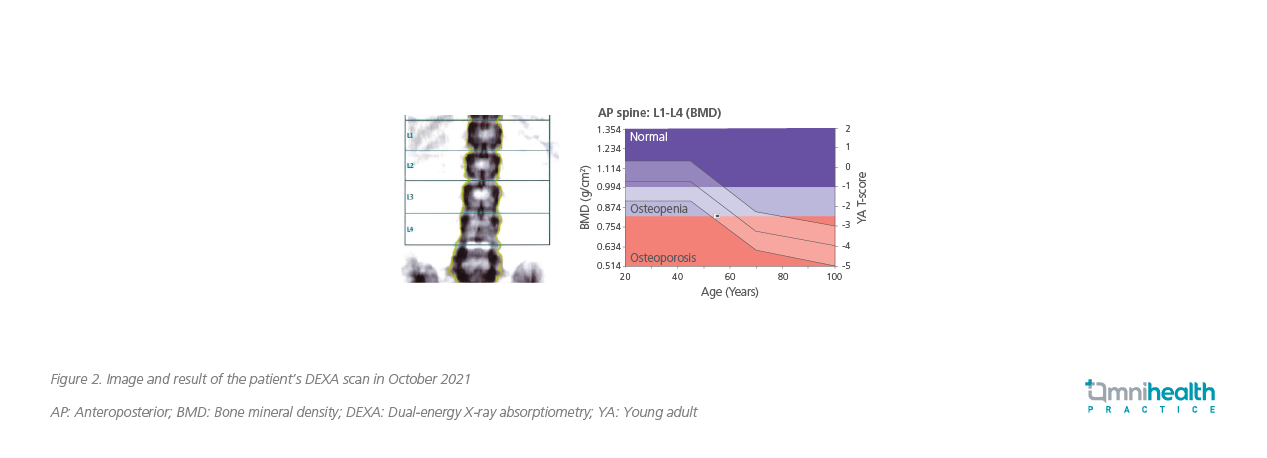
Overall, this patient responded well to romosozumab treatment which increased her average L1-4 BMD from 0.689g/cm2 to 0.818g/cm2 and her average T-score from -3.5 to -2.5 in 12 months (Figure 3). Given the importance of the use of antiresorptive agents to consolidate the BMD gains from the bone-forming agent, the patient continued with 6-monthly denosumab treatment after she had completed the 12-month romosozumab therapy.
The patient’s renal function, liver function, complete blood count, and serum calcium level were measured and were normal at baseline. All these parameters did not change during and after 12 months of romosozumab treatment. Her serum Vitamin D level was at a low level of 34.7nmol/L prior to the treatment, and she was prescribed vitamin D3 tablets 4,000 International Units (IU) per day for 30 days. The daily dose was then reduced to 1,000IU to maintain her serum Vitamin D level at 99.7nmol/L.
Romosozumab was well tolerated by this patient. She did not experience any major or serious adverse events during the treatment except for the mild injection-site reactions which resolved quickly after drug administration.
Discussion
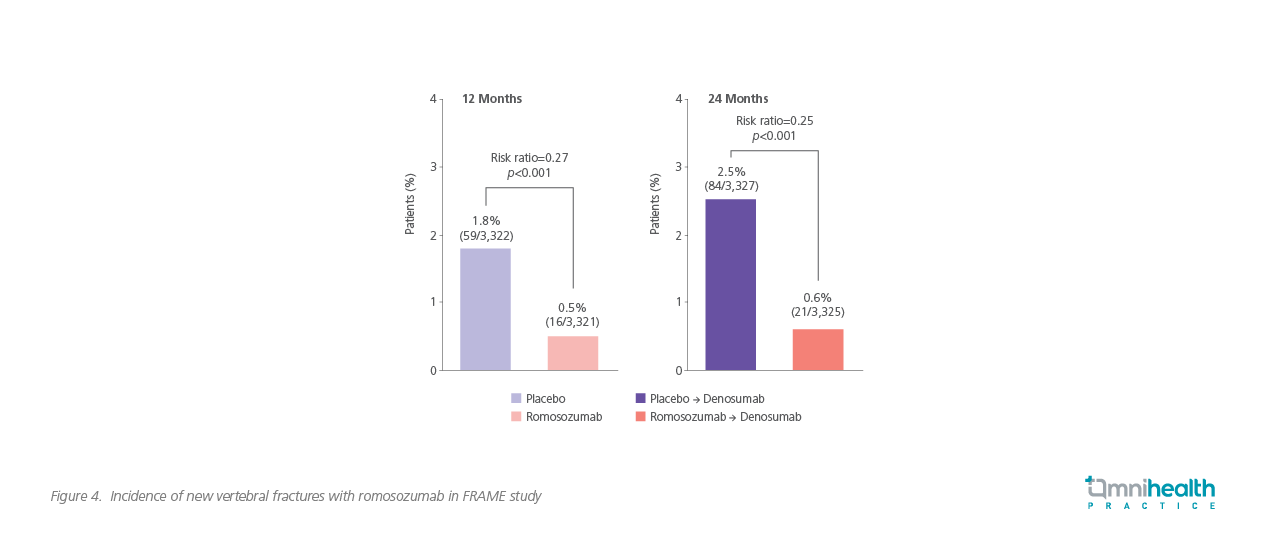
In 2016, a phase 3 clinical trial, called ‘Fracture Study in Postmenopausal Women with Osteoporosis’ (FRAME), evaluated the efficacy and safety of romosozumab in the post-menopausal osteoporosis women with T score of -2.5 to -3.5 at the total hip or femoral neck, followed by a maintenance therapy with a potent antiresorptive agent, denosumab.6 Romosozumab reduced the risk of new vertebral fractures by 73% compared with placebo (p<0.001) and increased bone mineral density (BMD) at the lumbar spine by 13.3% (95% CI: 11.9-14.7), at the total hip by 6.9% (95% CI: 5.6-8.1), and at the femoral neck by 5.9% (95% CI: 4.3-7.4) (p<0.001 for all comparisons).6 Even after transitioning to receive denosumab for the following year, BMD continued to increase and the difference in vertebral fracture rates persisted, with fewer vertebral fractures, in the romosozumab-to-denosumab group compared with placebo-to-denosumab group.6 At 24 months, the rates of vertebral fractures were significantly lower in the romosozumab-to-denosumab group (0.6% vs. 2.5%) than in the placebo group, and a 75% lower risk of fractures with romosozumab was observed (p<0.001) (Figure 4).6
This benefit was further proven in the 36-month extension study of FRAME, in which the relative risk reduction of new vertebral, clinical and nonvertebral fracture was 66%, 27% and 21% respectively in the romosozumab-to-denosumab group compared with placebo-to-denosumab group. BMD continued to increase for 2 years with denosumab treatment in both arms.13
Comparing with the clinical case, the 12-month romosozumab treatment resulted in an increase of BMD by 18.7% in this patient, higher than the result of 13.3% (95% CI: 11.9-14.7) found in the FRAME study. Based on the promising results of the study, it was convinced that the risk of new vertebral fractures in this patient has been greatly reduced. The patient has continued her osteoporosis treatment with the 6-monthly denosumab, and the long-term responses and benefits of romosozumab remain to be seen. Theoretically, the use of antiresorptive agents right after bone-forming agents would be beneficial to consolidate the BMD gain from romosozumab therapy. In fact, the benefit had been verified by the FRAME study in which romosozumab-treated patients had a 66% lower risk of new vertebral fractures after an additional 2 years of denosumab treatment.
Apart from the pharmacological treatment, adopting a “bone-friendly” lifestyle will be conducive to osteoporosis treatment and bone health. Patients are advised to include vegetables, whole grains, and calcium-rich foods in their diets. Regular exercises and outdoor activities are also indispensable. For patients without sufficient sun exposure, vitamin D supplements could be considered. In addition, since cigarette smoking, alcohol, and caffeine were shown to be associated with impaired bone metabolism, their consumption should be limited.
Conclusion/Messages to physicians
Increased screening for osteoporosis, timely pharmacological treatment, and adoption of a “bone-friendly” lifestyle are the mainstay to tackle the challenges of osteoporosis and its associated bone fractures arising from Hong Kong’s ageing population. In high-risk osteoporotic patients, bone-forming treatment is crucial for the reduction of their fracture risk. The rationale of deciding appropriate treatment for these patients is by assessing the risks and benefits, as well as by including patients’ preferences. For example, romosozumab could be a suitable treatment option for patients who do not have cardiovascular risk factors and value convenience. All in all, romosozumab is an effective and safe alternative which provides greater convenience to patients and should be considered among patients with postmenopausal osteoporosis.

