Shaping romosozumab treatment for postmenopausal osteoporosis: Balancing the fracture benefit and cardiovascular risk
As osteoporosis and cardiovascular (CV) diseases share an association between incidence and age, the CV implications of osteoporosis medications should be understood to clarify the potential mechanistic associations between the two conditions.1 In the previous ARCH and FRAME studies, romosozumab demonstrated clinical benefit in reducing fracture risk among postmenopausal women with osteoporosis.2,3 However, an imbalance of serious CV events was reported among the romosozumab-treated postmenopausal osteoporotic patients in the ARCH study, but not the FRAME study.2 In the World Congress on Osteoporosis, Osteoarthritis and Musculoskeletal Diseases, Professor Gaetano De Ferrari, Professor of Cardiology, The University of Torino, Italy, shared his insights on shaping romosozumab treatment in patients with osteoporosis by balancing the fracture benefit and CV risk.
Romosozumab demonstrated absolute clinical benefit in reducing fractures
Romosozumab is a monoclonal antibody that binds and inhibits sclerostin with a dual effect of increasing bone formation and decreasing bone resorption.4 The efficacy of romosozumab was studied in the pivotal FRAME and ARCH clinical trials among postmenopausal women with osteoporosis.
In the international, randomized, double-blind, placebo-controlled, parallel-group FRAME study, postmenopausal women were randomly assigned, in a 1:1 ratio, to receive romosozumab in a blinded fashion at a dose of 210mg monthly or placebo.2 At 12 months, there was a 73% lower risk for new vertebral fractures and a 36% lower risk of clinical fractures in the romosozumab group when compared to placebo (p<0.001).2 At 24 months, the rates of vertebral fractures were significantly lower in the romosozumab group than in the placebo group after both groups transitioned into denosumab treatment.23
In the phase 3, multicenter, international, randomized, double-blind ARCH study, postmenopausal women with osteoporosis who were at high risk for fracture were randomly assigned, in a 1:1 ratio, to receive monthly subcutaneous romosozumab at a dose of 210mg or weekly oral alendronate at a dose of 70mg for 12 months.3 Randomization was further stratified according to age by <75 or ≥75 years. In postmenopausal women, romosozumab treatment for 12 months followed by alendronate resulted in a significantly lower risk of fracture than alendronate alone.3 From the results of both FRAME and ARCH studies, Prof. De Ferrari commented that, “The clinical benefit of romosozumab was very obvious, especially in the important endpoint of vertebral fractures.”
Cardiovascular risk assessment of ARCH and FRAME
Whereas the incidence of the composite of CV death, myocardial infarction (MI) and stroke in the first year was identical between the romosozumab and the placebo arms in the FRAME study (HR=1.03; 95% CI: 0.62-1.72), a higher incidence was observed among patients in the romosozumab group in the ARCH study (HR=1.87; 95% CI: 1.11-3.14) (Figure 1).5 Notably, both the FRAME and ARCH studies included elderly patients (55-90 years of age) with those having high-risk CV disease not excluded from the baseline population.2,3 As such, during the first 12 months of the ARCH study, 50 of 2,047 (2.5%) patients in the romosozumab group compared with 38 of 2,046 (1.9%) in the alendronate group have experienced serious CV events, primarily MI and stroke.3
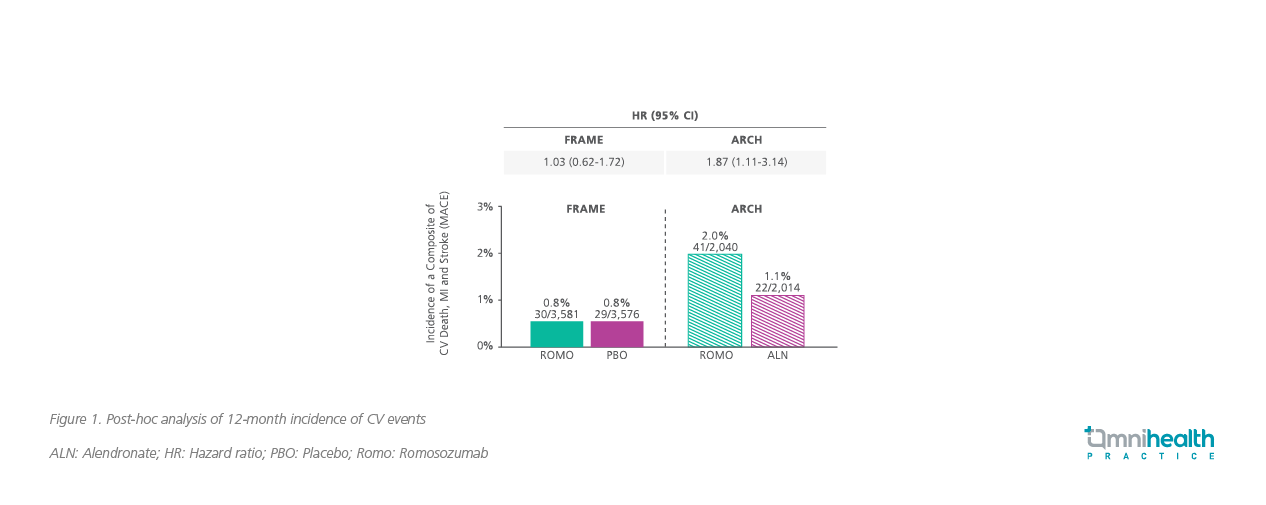
While patients in the romosozumab arm showed a greater cumulative incidence of major adverse cardiovascular event (MACE) than the alendronate arm in the ARCH study at 12 months (2.0% vs. 1.1%; HR=1.87; 95% CI: 1.11-3.14), it is remarkable that the cumulative incidence of MACE was similar between the alendronate arm in the ARCH study and the romosozumab and placebo arms in the FRAME study (Figure 2).5 Also unusual is the fact that the cumulative incidence of MACE in the alendronate group of the ARCH study was non-linear and showed a clear increase from 18 to 36 months.5
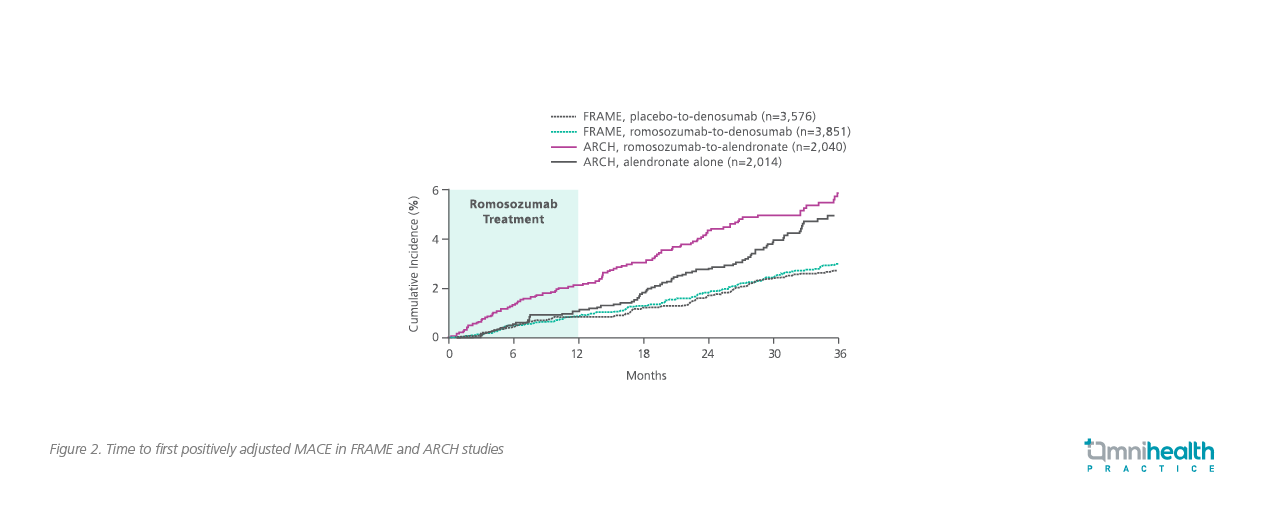
Indeed, when accounting for the overall study period of both FRAME and ARCH studies, the incidence of serious CV adverse events was generally balanced between the romosozumab and alendronate arms (HR=1.13; 95% CI: 0.93-1.38).5 In terms of adjudicated serious CV adverse events, the alendronate arm of the ARCH study actually had a similar incidence when compared to the romosozumab arm in the FRAME study (1.9% vs. 1.2%).2,3 Where a difference in incidence rate of CV death, MI and stroke at 12-months was observed in the ARCH study, the difference was actually not clinically relevant (0.8% in the romosozumab arm vs. 0.6% in the alendronate arm).3 Given the unusual behavior and the low actual number of CV events observed in the ARCH study, Prof. De Ferrari emphasized “When considering the individual endpoints for stroke and MI, each endpoint had less than 1% of incidence rate. Therefore, the accuracy of the estimates was limited by the low number of events.”
No direct association between increased CV risk among patients with CV risk factors
In the subsequent subgroup analysis of the FRAME and ARCH studies, the relative risk of CV events was generally consistent in the 12-month period between the romosozumab and control groups.5 Particularly in the ARCH study, no specific CV risk factor such as age, smoking history or prior history of MI and stroke, was found to be associated with an increased relative risk of MACE among patients receiving romosozumab versus patients receiving alendronate (Figure 3).5
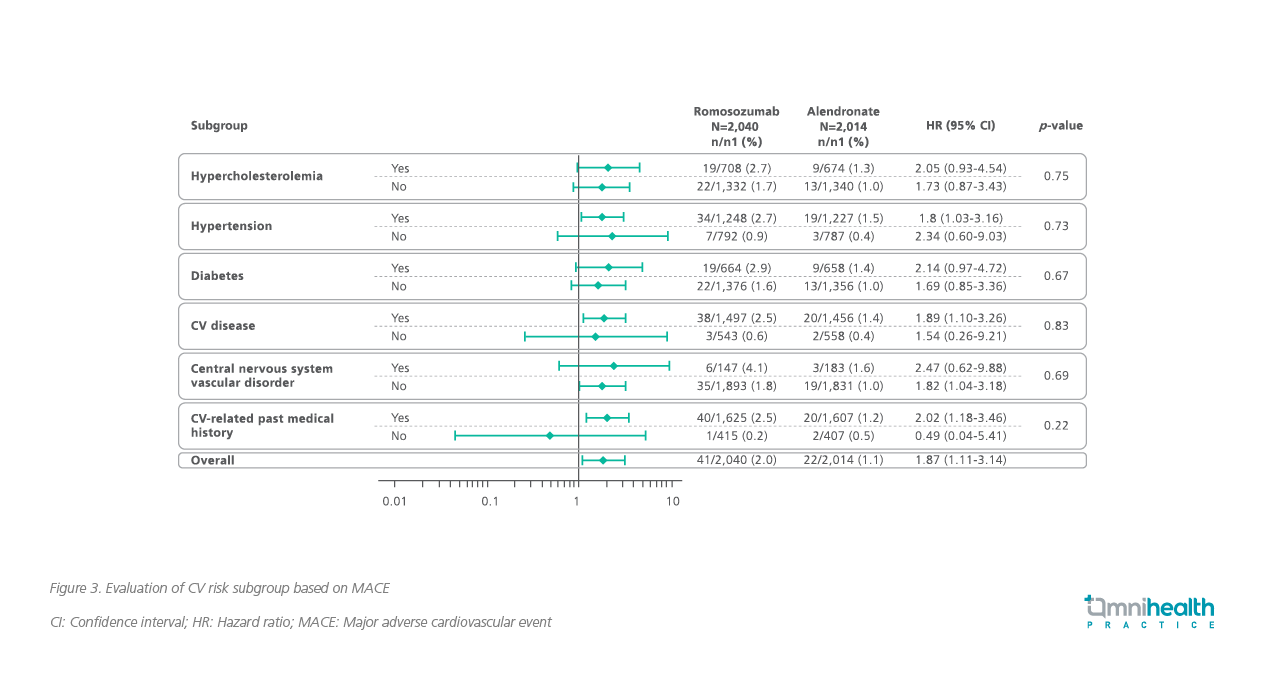
However, when considering the absolute risk, the possibility of MACE in patients with a prior MI or stroke was higher.5 Among patients with prior MI or stroke, a 12-month MACE incidence rate of 3.6% and 2.1% were reported in the romosozumab and alendronate arms, respectively, versus 1.1% and 0.8% in those without a prior history.5 In the subgroup analysis of the overall study period, the absolute risk for MACE increased to 8.9% and 5.8% in the subjects with prior MI or stroke in the romosozumab arm and alendronate arm, respectively, compared to 3.5% and 3.2% in those without a prior MI or stroke.6 Prof. De Ferrari acknowledged that these findings suggested the opportunity of contraindicating use of romosozumab in patients with a prior history of MI or stroke.
Patients with CV conditions were not excluded from ARCH and FRAME
During the first 12 months of the ARCH study, the incidence of MACE reported in the romosozumab arm was doubled as compared to the alendronate arm.5 These higher CV outcomes were exclusively found in the ARCH study which may be attributed to the ARCH population having a higher prevalence of baseline CV risk factors than the FRAME population. Indeed, patients in ARCH were older, had more prevalent hypertension, cerebrovascular conditions, ischemic heart disease, heart failure, and atrial fibrillation.5
According to data from the World Health Organization, survivors of MI are at increased risk of recurrent CV events and have an annual death rate of five-six times higher than people of the same age who do not have coronary heart diseases. Similarly, patients who have suffered a stroke remain at an increased risk of a further stroke (about 7% per annum).7 Prof. De Ferrari explained, “Indeed there was a higher baseline risk profile in the ARCH study, and those higher baseline risk factors have accounted for higher CV incidence rate observed.”
No CV risk related to romosozumab was found
Due to the reports of increased CV events in the ARCH study, further genetic, clinical and non-clinical studies were conducted to assess the effects of sclerostin inhibition on the CV system.8 However, these studies did not find any functional, morphological or transcriptional effects on the CV system in animal models with sclerostin suppression.8 In addition, the nonclinical studies did not identify evidence that could demonstrate any association between sclerostin inhibition and adverse CV function, increased CV calcification or atheroprogression.8
Also, romosozumab or recombinant sclerostin did not have any effect on vasoconstriction or platelet aggregation.8 The absence of sclerostin did not result in macroscopic or microscopic evidence of vascular abnormalities or mineralization in 6-month, repeatdose toxicity studies in monkeys.4,5 Similarly, no exacerbation of spontaneous age-related vascular calcification characterized by focal medial calcification or ectopic ossification was found in rat models following lifetime exposure to romosozumab despite the 19 times longer exposure when compared to the typical clinical exposure duration.4,5
Most importantly, as demonstrated in a meta-analysis of 17 randomized controlled trials, romosozumab treatment did not increase the risk of composite CV outcomes, 3-point MACE and specific CV events. However, a chance of 4-point MACE increase among elderly men and postmenopausal woman with osteoporosis over a period of 12-36 months was observed.10
The potential risk of CV events should be balanced against the benefit of reduced fracture risk
According to the European Medical Agency (EMA) recommendations, when determining whether to use romosozumab for an individual patient, consideration should be given to the patient’s fracture risk over the next year and CV risk based on risk factors (e.g., established cardiovascular disease, hypertension, hyperlipidemia, diabetes mellitus, smoking, severe renal impairment, age).11 The recommendations also mentioned that if a patient experiences a MI or stroke during therapy, treatment with romosozumab should be discontinued.11
Prof. De Ferrari continued, “The clinical benefit of romosozumab is considered to outweigh the risk of adverse CV events in patients with severe postmenopausal osteoporosis at high risk of fracture without a previous MI or stroke.” In fact, less limitations are suggested by the American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE) clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis that recommend the use of romosozumab except in patients with a recent MI or stroke.12,13
In accordance with the positive findings shown by romosozumab in the FRAME and ARCH studies, both the EMA and AACE/ACE guidelines have recognized the clinical benefit of romosozumab and recommended romosozumab for clinical use after balancing the CV risk. “The benefitrisk balance of romosozumab should be discussed fully with each patient and should only be used if it is agreed that the benefits outweigh the risks,” Prof. De Ferrari concluded.
Conclusion
In the FRAME and ARCH clinical studies, romosozumab has demonstrated superior fracture reduction benefit in patients with postmenopausal osteoporosis. Notably, an excess of serious CV events was observed in the ARCH study between the romosozumab and the alendronate arms in the first 12 months of treatment but was not present at the end of follow-up. There are uncertainties in respect to the interpretation of CV data when considering that the ARCH study was not designed or powered for CV endpoints. Overall, very few CV events were observed in both the FRAME and ARCH studies. Also, none of the preclinical or clinical studies have demonstrated a significant CV risk with the use of romosozumab. Given its benefit-risk assessment, romosozumab should be utilized in the treatment of patients with postmenopausal osteoporosis without a prior history of MI and stroke.
Two well-known diabetes drugs similarly effective in reducing heart and kidney disease
Glucagon-like peptide-1 receptor agonists (GLP-1 RA) and sodium-glucose co-transporter 2 inhibitors (SGLT2i ), which are commonly used to treat type 2 diabetes mellitus (T2DM) are similar in their ability to reduce major heart complications, including heart attack, stroke and death from cardiovascular disease. These findings were accepted for presentation at the Endocrine Society’s Annual Meeting 2020 (ENDO 2020).1,2

