MEETING HIGHLIGHT
Implication of the latest COPD guidelines in the care of elderly patients
Chronic obstructive pulmonary disease (COPD) used to be common, untreatable, unresponsive, and relentlessly progressive, leading to high morbidity and mortality worldwide.1 However, with advances in treatments and guidelines over the decade, COPD has now become a preventable and treatable disease. At the recent webinar on COPD organized by the Hong Kong Geriatrics Society, Dr. Richard E K Russell presented his insights on the latest COPD guidelines, stressing the importance of individualized COPD treatment according to patient characteristics, in addition to the adoption of dual bronchodilators - long-acting muscarinic antagonist (LAMA) plus long-acting β2-agonist (LABA) - as initial treatment for all COPD patients, especially for those with dyspnea or exercise intolerance.
Current COPD treatment landscape and gap
COPD is a common, preventable and treatable disease. Yet, it still poses great public health challenges, given the increasing morbidity and mortality worldwide.1 COPD is characterized by persistent respiratory symptoms, including dyspnea, cough, sputum production, and airflow limitation, due to airway and/or alveolar abnormalities.1 Tobacco smoking remains the main risk factor for COPD, but other environmental and host factors, such as genetic abnormalities, abnormal lung development, or aging may predispose individuals to COPD development.1
As advised by the 2021 Global Initiative for Chronic Obstructive Lung Disease (GOLD), the treatment goal for COPD is to control symptoms and progression, improve exercise tolerance and health status, prevent and treat exacerbations, as well as reduce mortality.1 Early COPD diagnosis based on symptoms, risk factors and spirometry, followed by an assessment to determine the GOLD stages based on symptoms and exacerbation history, is of utmost importance.1 Initial management should include non-pharmacological treatments, such as smoking cessation, vaccination, increasing exercise, and controlling comorbidities, along with pharmacological interventions.1
With regard to the current treatment landscape of COPD, the most widely used therapy is the inhaled corticosteroid (ICS)/ long-acting β2-agonist (LABA), irrespective of the disease severity and similarity. A database study assessing 24,957 COPD patients irrespective of GOLD stage and group examined the use of COPD medication, with 17% receiving no treatment, 24% receiving ICS, 26% receiving ICS/LABA, and 23% receiving ICS, LABA, long-acting muscarinic antagonist (LAMA), and 2% receiving LAMA alone.2 Similarly, a retrospective study of the United Kingdom (UK) general practice showed that 63% of 29,815 COPD patients of GOLD stage A or B were treated with ICS-containing treatment at diagnosis.3 In view of the dominant usage of ICS/LABA, which may not be an ideal option, a personalized treatment is essential to COPD patients.
Greater benefits of dual bronchodilators as initial treatment
“Long-acting therapy is better than short-acting therapy, LAMA is better than LABA, and two - LAMA plus LABA (dual bronchodilators) - is better than one,” emphasized Dr. Russell. Long-acting bronchodilators are the cornerstone of therapy with LAMA preferred due to its superior effects on exacerbations.1 According to the GOLD guidelines, there is no role of ICS-containing treatment in GOLD groups A, B and C (Figure 1).1 ICS/LABA may be considered as initial therapy for GOLD group D only if eosinophil count is ≥300cells/µL.1
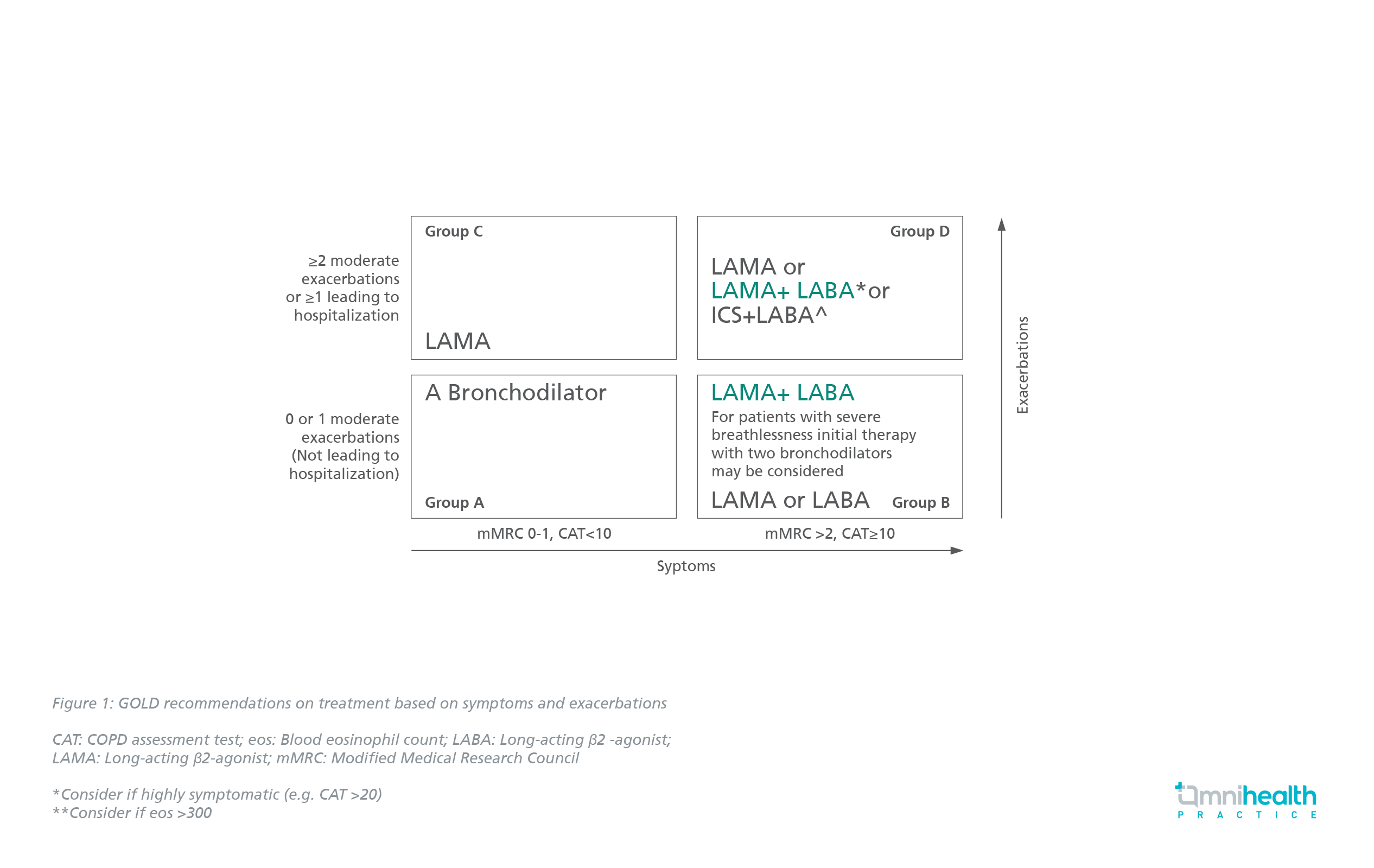
More importantly, an increase in lung function, as well as a reduction in symptoms and exacerbations is best achieved with LABA and LAMA combinations.1
The 2020 American Thoracic Society (ATS) guidelines also state that dual therapy of LAMA and LABA helps reduce hospitalization by 11% and exacerbations by 20%, compared with monotherapy.4 The 2019 UK National Institute for Health and Care Excellence (NICE) emphasizes the use of LAMA/LABA in all patients followed by LABA/LAMA/ICS in case of no improvement, and LABA/ICS is only considered in specific COPD patients.5
The benefits of dual therapy have been further proven in a post hoc analysis of the TONADO trials, which analyzed data from 2,055 patients treated with either tiotropium/olodaterol (T/O) 5/5μg or tiotropium (Tio) 5μg (Delivered via Respimat®) in 2 replicate, 52-week, parallel-group, double-blind studies.6 Results showed that the T/O treatment significantly increased the time to, and reduced the risk of clinically important deterioration (CID) versus Tio in all populations (Median time to CID: 226 vs. 169 days) (HR=0.76; 95% CI: 0.68-0.85; p<0.0001) (Figure 2).6
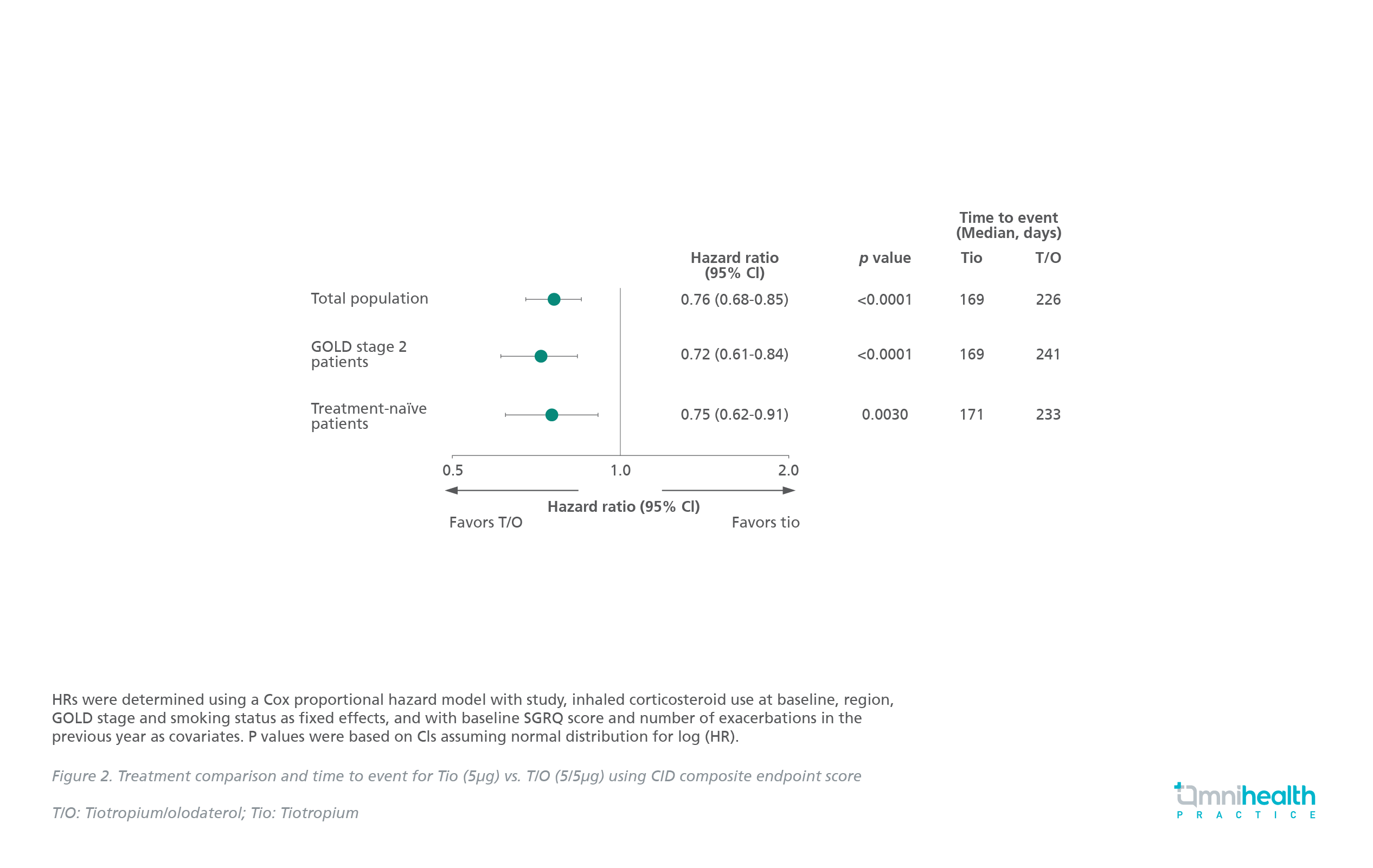
These demonstrated the advantages of treatment optimization with T/O over Tio monotherapy in delaying and reducing the risk of CID in patients with early COPD.6
In the OTEMTO study, T/O combination also demonstrated significant improvement in lung function, transitional dyspnea index (TDI), quality of life (QoL), and physical activity at week 12 in COPD patients including those in GOLD 2.7
Studies show superiority of LABA/LAMA over LABA/ICS
Multiple studies have shown the superiority of LAMA/LABA over LABA/ICS, in terms of efficacy and safety. The CITRUS study, a real-world Korean study, compared the escalation time to triple combination therapy of LAMA and LABA/ICS from LAMA or ICS/LABA initiation in COPD. Results demonstrated a higher incidence of treatment escalation (p<0.0001) and pneumonia in patients receiving ICS/LABA compared with LAMA (147.89 vs. 90.20 per 1,000 person years).8,9
Likewise, the ENERGITO trial assessed the change in the 24h FEV1 6 weeks after treatment, which showed a significantly increased lung function with T/O compared with salmeterol/fluticasone therapy.10 A Cochrane database review also showed an improvement in trough FEV1, QoL, and breathlessness with dual bronchodilator therapy.11,12
Moreover, a meta-analysis demonstrated that LABA/LAMA treatment was associated with significant reduction in moderate/severe exacerbations and pneumonia compared with LABA/ICS.13 These results were driven by the FLAME study that highlighted the superiority of dual bronchodilators over LABA/ICS.13
In a US real-world study, patients on T/O had 77% less escalation to the triple therapy, 24% less COPD exacerbations, and 26% less pneumonia requiring hospitalization than patients on LABA/ICS irrespective of the baseline eosinophils and exacerbation history.14
ICS-containing treatments limited to certain COPD patients
GOLD recommends ICS use in specific COPD GOLD D patients with high risk of exacerbations and elevated blood eosinophil levels to maximize the benefits while minimizing the risks.1 Bronchodilators are recommended in most COPD patients with dyspnea, and only a few of those with exacerbations with or without ICS.1 Patients most likely to be benefited from the triple therapy are those with a blood eosinophil count ≥ 300cells/μL or ≥2 exacerbations per year, or those with persistent breathlessness or exercise limitations, despite LAMA/LABA or LABA/ICS treatment.1
Since there is no clear evidence of benefits but potential harm, the use of ICS is not recommended with blood eosinophil count <100cells/μL, recurrent pneumonia, or with history of mycobacterial infection.1 It may be considered individually in patients with 1 moderate COPD exacerbation per year and blood eosinophils of 100-300cells/μL.1
The ATS guidelines recommend triple therapy over dual therapy in certain COPD patients with dyspnea or exercise intolerance, who had 1 or more exacerbations in the past year requiring antibiotics, oral steroids, or hospitalization.4
A pooled analysis from six phase 3/4 trials, which included patients with mild to very severe COPD and predominantly low risk of exacerbations, demonstrated no difference in the risk of all-cause mortality time between patients on LAMA/LABA or LAMA/LABA/ICS (Figure 3).15

ICS withdrawal recommended in stable patients
Multiple studies have shown that COPD patients are at a higher risk of experiencing side effects, such as pneumonia, oropharyngeal candidiasis, hoarseness, skin bruising, tuberculosis, cataracts, diabetes, and fracture.1
Patients with no exacerbations in the previous year may be the candidates for ICS withdrawal, with no significant increase in the risk of exacerbation, based on the ATS guidelines.4 The ERS guidelines also recommend ICS withdrawal in patients with no history of frequent exacerbations and switching to one or two long-acting bronchodilators. However, patients with blood eosinophil count ≥300cells/μL irrespective of exacerbation history should remain on ICS, while those with frequent exacerbations and eosinophil count <300cells/μL should be assessed individually for their continued need of ICS due to the limited evidence.16
Data from two parallel randomized trials have shown the correlation between eosinophils and ICS efficacy in COPD, where patients with the highest eosinophil count on ICS had the greatest reduction in exacerbation frequency.17
The WISDOM study compared patients who entered a 6-week run-in period of triple therapy, remained on ICS or had a stepwise ICS withdrawal to dual bronchodilators.18 ICS withdrawal did not increase the risk in time to exacerbation, although there was a clinically insignificant decrease in lung function.18
Furthermore, the DACCORD study compared disease progression in patients receiving dual bronchodilation or triple therapy in the real-world population, which has demonstrated limited benefit in decreasing the exacerbation rate.19
Respimat® as an optimal inhaler device for all COPD patients
The NICE guidelines state the importance of selecting the appropriate inhaler device based on the patient’s individual need for the sake of achieving optimal control and preventing future risk.5 Effective COPD treatment requires that the inhaled drug reaches the lower airways to achieve the desired therapeutic effect. The penetration of a respiratory drug into the airways is influenced by 4 key inhaler characteristics: Aerosol velocity, duration, particle size, and internal device resistance.20
Different inhalers have varying lung deposition rates, with Respimat® having the highest rate (51.6%), followed by Turbohaler (28.5%) and Becloforte (8.9%). 21 Respimat® inhaler delivers a consistent dose and achieves the highest flow rate with the least inspiratory effort.22
The randomized, placebo-controlled TRONARTO study was conducted to assess the effectiveness of soft mist inhaler, Respimat® in COPD patients stratified by the peak inspiratory flow (PIF).23 A total of 213 patients were randomized to receive tiotropium/olodaterol (5μg/5μg) or matched placebo delivered via Respimat®. The endpoints included FEV1 AUC0-3h and trough FEV1.23 Results of this study have demonstrated similar FEV1 AUC0-3h and trough FEV1 levels in the groups of patients with PIF <60 L/min and PIF ≥60L/min, indicating that Respimat® made similar improvement in lung function independent of patients’ inhalation abilities (PIF <45-≥60L/min) and COPD severity.23
Conclusion
It is advised that COPD should be diagnosed early, along with the importance of smoking cessation and lifestyle interventions.1 LAMA/LABA is the preferred pharmacological therapy in almost all COPD patients, except few patients who may be the candidates for ICS-containing therapy.1 Regular assessment of the patient’s symptom scores and exacerbation risk is required for consideration of ICS use or withdrawal.4 The optimal inhaler device that delivers consistent and desired therapeutic effects should be adopted, based on the patient’s individual abilities and preferences, in order to improve the treatment outcomes.1

