CASE REVIEW
Dual immunotherapy combination in pre-treated HCC: A case study in achieving 10-year remission
Hepatocellular carcinoma (HCC) remains one of the most challenging cancers to treat globally, often being diagnosed at an advanced stage and associated with poor prognosis.1 Historically, treatment options such as tyrosine kinase inhibitors (TKIs) like sorafenib have offered only modest survival benefits, underscoring the urgent need for more effective therapies.2 The emergence of dual immune checkpoint inhibitors has revolutionized the therapeutic landscape of advanced HCC (aHCC).2 In an interview with Omnihealth Practice, Professor Yau, Chung-Cheung Thomas shared a case of 10-year survival in a patient with metastatic aHCC in the second-line setting managed with dual immune checkpoint inhibitors nivolumab + ipilimumab. Dr. Wong, Sum-Lung Jeffrey also provided insights into data from the CheckMate 040 trial and its Asian subgroup analysis, highlighting not only the efficacy and durability of the dual immune checkpoint inhibitors but also their manageable safety profile. The combination of nivolumab + ipilimumab marks a paradigm shift in HCC treatment, offering hope for long-term survival in a disease once considered universally fatal.
Background
Advanced HCC: A combination approach
Treatment for aHCC has historically relied on TKIs, and in the post-sorafenib setting, second-line TKIs achieve only modest survival benefits of around 10 months.3,4 Immune checkpoint inhibitors, such as anti-programmed cell death protein 1 (PD-1) like nivolumab, and anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) like ipilimumab, work in tandem to stimulate the body’s anti-tumor immune response.1 Dr. Wong noted the complementary actions of the two drugs, which target different phases of T-cell activation, with anti-PD-1 primarily acting at the effector phase and anti-CTLA-4 at the T-cell activation and proliferation phases.1 The efficacy of this potent combination of nivolumab + ipilimumab in aHCC is illustrated by a case study shared by Prof. Yau.
Case sharing
The patient was a 50-year-old male with extensive metastatic aHCC diagnosed in 2015. He presented with mediastinal, peritoneal and bone metastases, and had progressed rapidly on the only standard therapy at the time, sorafenib, without evidence of stabilization or response. The introduction of ipilimumab 3mg/kg and nivolumab 1mg/kg every 3 weeks for 4 cycles, followed by nivolumab 240mg every 2 weeks, marked a significant turning point, with the ambitious goal of achieving long-term survival despite his metastatic disease.
The therapy demonstrated a profound and rapid effect. Despite initial pseudoprogression on scans, the patient's primary tumors shrank by nearly half on subsequent computed tomography (CT) scans, and his alpha-fetoprotein (AFP) levels experienced a spectacular drop from over 20,000 to around 100 within the first month. The patient went on to achieve partial and then complete remission, with his AFP levels fully normalizing in three months. CT scans revealed sustained remission over the years (figure 1).
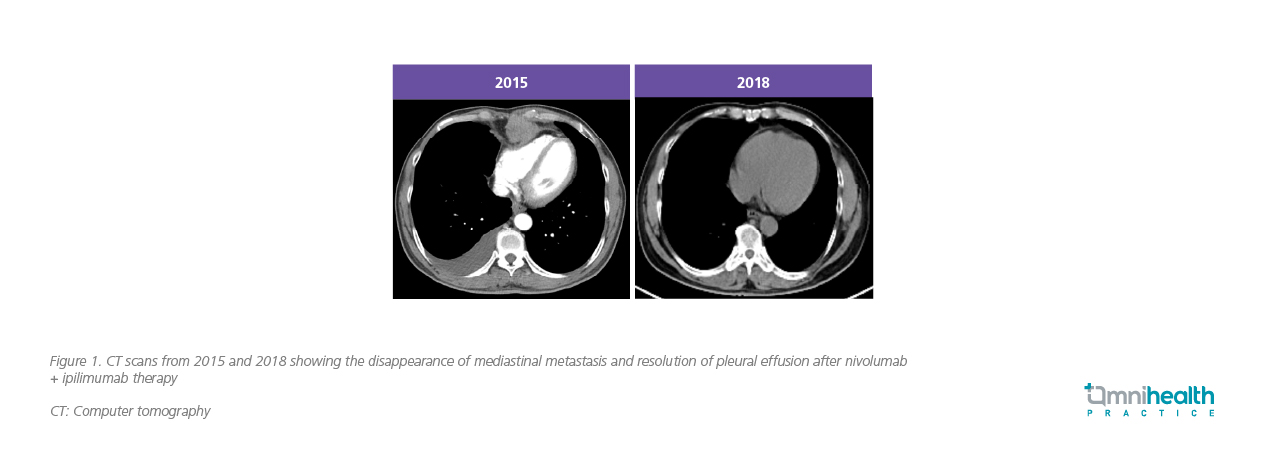
He successfully continued treatment for over four years, receiving more than 100 cycles of therapy. The patient had hypophysitis, which is commonly seen in immune checkpoint inhibitor therapy, and was given hydrocortisone supplements. He also had mild pruritus. Otherwise, the therapy was well-tolerated.
Discussion
Rewriting 5-year survival with nivolumab + ipilimumab in CheckMate 040
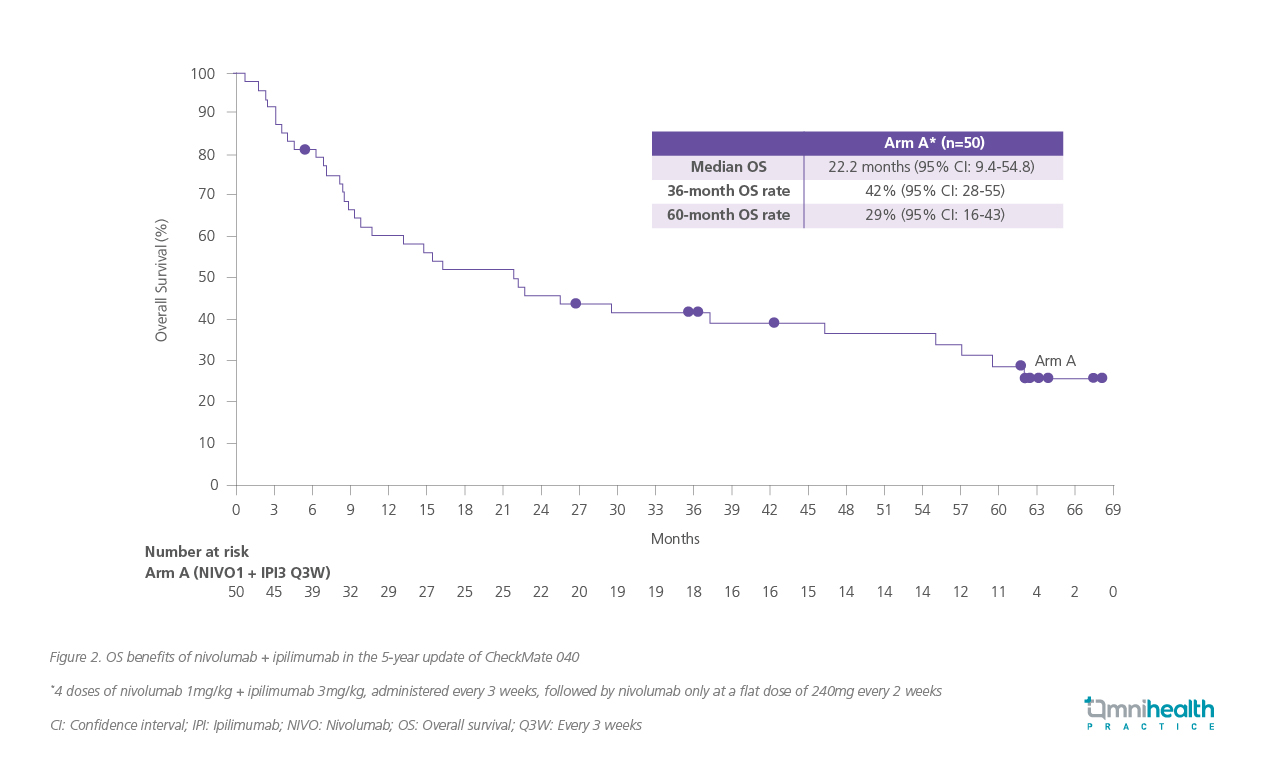
The extended survival observed in this patient corroborates findings from the CheckMate 040 study. Led by Prof. Yau, this global study demonstrated that the combination of nivolumab + ipilimumab provides durable, long-term clinical benefits for patients with aHCC.2 The CheckMate 040 trial was a phase 1/2, open-label study evaluating the efficacy and safety of the combination in adults with aHCC previously treated with sorafenib.1 In arm A of the study, patients received 4 doses of nivolumab 1mg/kg + ipilimumab 3mg/kg, administered every 3 weeks, followed by nivolumab only at a flat dose of 240mg every 2 weeks.1 The study's co-primary endpoints were overall response rate (ORR), safety, and tolerability, with duration of response (DOR) also assessed as a key outcome.1
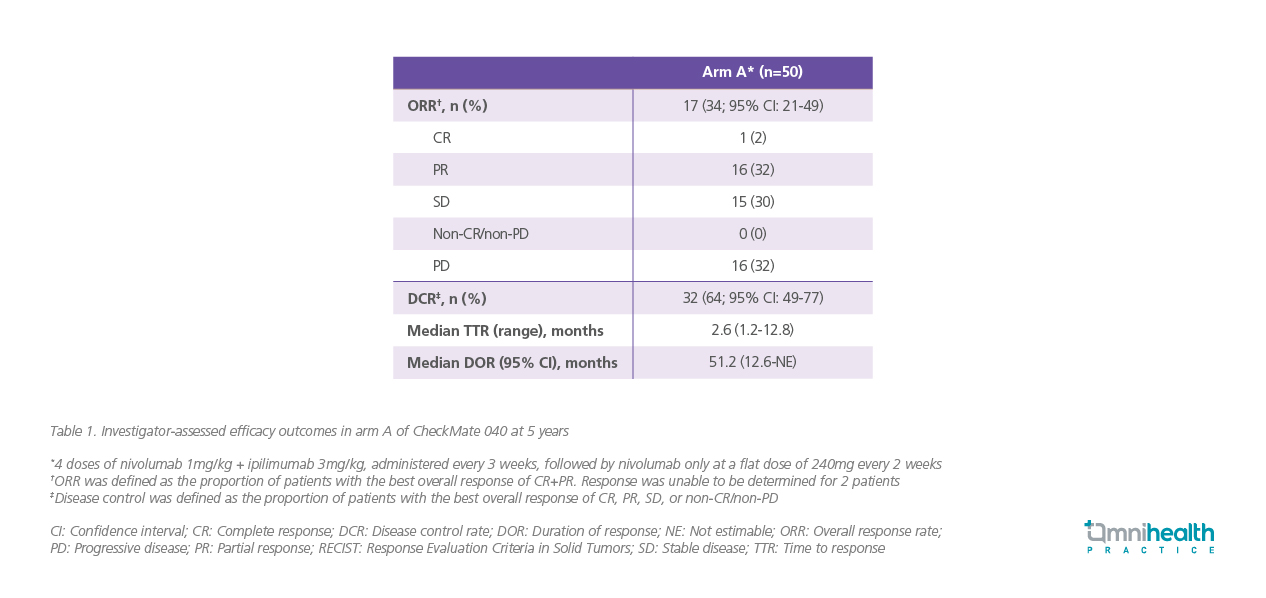
At a minimum follow-up of 60 months, the median overall survival (OS) was 22.2 months (95% CI: 9.4-54.8) in arm A.2 Landmark survival rates were 42% at 36 months (95% CI: 28-55) and 29% at 60 months (95% CI: 16- 43) (figure 2).2 Patients in arm A demonstrated an investigator-assessed ORR of 34% (95% CI: 21- 49), while the median DOR was 51.2 (95% CI: 12.6 -not estimable [NE]).2 A high proportion of patients achieved persistent disease control (table 1).2 These outcomes further demonstrated the long-term benefits of nivolumab + ipilimumab.2
Through the Asian lens: CheckMate 040 subgroup analysis
A subgroup analysis led by Prof. Yau also demonstrated the combination's effectiveness specifically for Asian patients.5 Asia, particularly Eastern and Southeast Asia, has a higher incidence of HCC.5 A crucial factor complicating HCC treatment is the notable heterogeneity between Asian and non-Asian regions.5 This divergence has been linked to different treatment outcomes, as seen in pivotal trials for agents like sorafenib and regorafenib, which showed varied survival rates between Western and Asian populations.6 Based on a sub-analysis of the CheckMate 040 study, the combination demonstrated durable efficacy in Asian patients that was comparable to the global cohort.5 In arm A, the blinded independent central review (BICR)-assessed ORR was 24% in the Asian cohort and 32% in the global cohort.5 Similarly, the disease control rate (DCR) was 50% in Asian patients and 54% in the global cohort.5 Long-term benefit was similarly shown, with a median OS of 16.4 months (95% CI: 8.6-not reached) for the Asian cohort.5
Mitigating IRAE anxiety while optimizing efficacy
Both analyses of CheckMate 040 detailed the safety profile of the combination.2,5 Dr. Wong highlighted that “The onset and pattern of immune-related adverse events (IRAEs) is unpredictable, but in general they are very manageable. In addition, having a mild IRAE is not necessarily a bad thing: In many tumor types, the development of an IRAE has been shown to correlate with response and survival. We explain this to patients while also managing any side effects cautiously.”
In CheckMate 040, nivolumab + ipilimumab demonstrated a manageable and consistent safety profile with five years of follow-up.2 Most patients received nivolumab + ipilimumab at a relative dose intensity of ≥90% with no new safety signals being identified.2
IRAEs were mostly low-grade, with the most common being rash, hepatitis, and adrenal insufficiency in both the Asian and global cohorts.5 Higher instances of immune-related rash were reported in the Asian population, but the rates of immune-related hepatitis were similar.5 Serious treatment-related adverse events (TRAEs) of grade 3 or 4 were reported in 20% of patients in arm A.2 The most frequent grade 3 or 4 IRAE was hepatitis, which was reported in 20% of patients in the global cohort and 21% in the Asian cohort.5
Dr. Wong shared that IRAEs are often manageable with vigilant monitoring and timely intervention. The development of IRAE may even correlate with better outcomes, reinforcing the need for a balanced approach to treatment. In his experience, close monitoring is essential for managing IRAEs. “We ensure our patients are well-informed, providing them with a hotline to report any symptoms promptly. Before each cycle, we conduct thorough blood work, checking complete blood count (CBC), liver and renal function tests (LFTs and RFTs), AM cortisol, and thyroid function.”
While endocrine IRAEs are generally manageable, they require vigilance for subtle signs.7 For example, patients should be explicitly warned that sudden fatigue could signal hypophysitis, a side effect that is easily missed but potentially dangerous.7 Additionally, if a liver-related IRAE is suspected, it is crucial to first exclude other causes like hepatitis flares or disease progression.7 For suspected severe IRAE, standard practice is to promptly start corticosteroids at a dose of 1mg/kg of prednisolone.7 Dr. Wong recalled, most IRAEs responded quickly and dramatically to steroid treatment. Once IRAE is controlled, the goal shifts to tapering immunosuppressants as rapidly and safely as possible to avoid compromising the anti-tumor response.7
Conclusion
The case presented by Prof. Yau and the insights from Dr. Wong powerfully illustrated that dual immunotherapy has established a new standard of care in advanced HCC. The combination of nivolumab + ipilimumab offers a genuine prospect of long-term remission, with its profound efficacy supported by data from the CheckMate 040 trial and its Asian subgroup analysis.2,5 Translating this promise into consistent clinical success hinges on a committed foundation of multidisciplinary care and thorough patient education to guide patients towards the best possible therapeutic outcomes.

