CONFERENCE UPDATE: EHA 2025
Sustained efficacy of fixed-duration ibrutinib + venetoclax in CLL/SLL: Final analysis of the phase 2 CAPTIVATE study
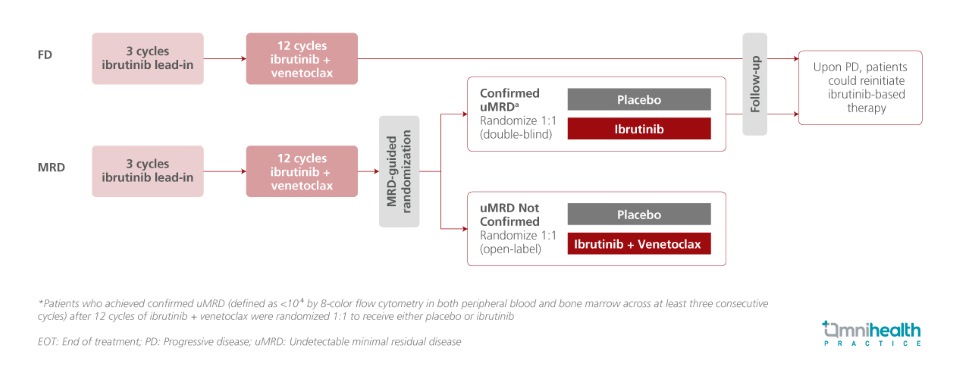
STUDY DESIGN
The phase 2 CAPTIVATE study evaluated the first-line use of all-oral, once-daily ibrutinib (a BTK inhibitor) + venetoclax (a BCL-2 inhibitor) in patients with chronic lymphocytic leukemia (CLL)/ small lymphocytic lymphoma (SLL), across two cohorts: a minimal residual disease (MRD)-guided randomized discontinuation cohort and a fixed-duration (FD) cohort.1 In earlier analyses with up to 5.5 years of follow-up, the FD regimen demonstrated sustained progression-free survival (PFS) at the 5-year landmark, including among patients with high-risk genomic alterations.1 With up to 7 years of follow-up, the final analysis of this phase 2 trial provides long-term outcomes, with a primary focus on the FD cohort.1
The study enrolled previously untreated CLL/SLL patients aged ≤70 years (n=202).1 All participants received a three-cycle lead-in of ibrutinib monotherapy (420mg once daily), followed by twelve cycles of combined ibrutinib (420mg once daily) and venetoclax (oral ramp-up over five weeks to 400mg once daily).1 Focusing on the FD cohort and the placebo arm of the MRD-guided cohort, all patients in the FD cohort (n=159) completed the planned 15-month regimen and discontinued therapy, while patients in the MRD cohort placebo arm with confirmed undetectable minimal residual disease (uMRD) (n=43) received one additional cycle of ibrutinib + venetoclax during randomization, followed by placebo treatment.1 Among patients with confirmed progressive disease (PD), a total of 36 who met iwCLL criteria for retreatment received either single-agent ibrutinib (n=25) or fixed-duration ibrutinib + venetoclax (n=11).1 Notably, in both the total pooled population and the FD cohort, approximately 60% of patients harbored high-risk unmutated IGHV (uIGHV), while del(17p) or TP53 mutations─ also considered high-risk genomic alterations were present in 14% of the total population and 17% of the FD cohort.1
Key outcomes assessed included PFS, overall survival (OS), impact of del(17p)/mutated TP53 and IGHV status on long-term PFS, and time to next treatment (TTNT) with up to 7 years of follow-up.1
FINDINGS
| Efficacy outcomes : |
|
|
|
|
|
|
| Safety : |
|
|

