CONFERENCE UPDATE: ESMO 2024
Development of a TR-TRM model as a risk prediction tool for severe toxicities in elderly cancer patients on systemic treatment
Older cancer patients face an elevated risk of severe treatment-related toxicities (TRT), partly due to age-related physiological changes and comorbidities.1 However, existing tools for predicting chemotherapy toxicity have limitations in both development and practical use.1 This local study aimed to develop and validate a prediction model specifically for severe TRT in older cancer patients receiving systemic treatments.1 Dr. Chan, Wing-Lok Wendy from the Department of Clinical Oncology at the Li Ka Shing Faculty of Medicine, University of Hong Kong, presented these findings at the ESMO Congress 2024.1
The study recruited consecutive cancer patients aged 65 and above from three large oncology centers in Hong Kong (Queen Mary Hospital, Queen Elizabeth Hospital, and Gleneagles Hospital) between March 2019 and May 2022.1 The pre-treatment evaluation included clinical, tumor/treatment, laboratory, and geriatric assessments.1 Patients were monitored throughout their systemic anti-cancer treatment or for up to six months to document any grade 3-5 TRT according to CTC-AE version 5 criteria.1 The model was developed using univariate and multivariable logistic regression to identify predictive factors, with a weighted scoring system to assess risk (table 1).1 Model performance was evaluated using the area under the ROC curve (AUC) and Hosmer-Lemeshow’s goodness-of-fit test, with both internal and external validation.1
Of the 500 participants (400 in the development cohort, 100 in the validation cohort), the median age was 71 (range 65-96), and 304 (60.8%) experienced grade 3-5 TRT.1 Patient characteristics were similar between the development and validation cohorts, with a balanced gender distribution (52.2% male).1 Most patients received palliative treatment (66.2%) compared to radical/adjuvant therapy (33.8%) and were treated with chemotherapy (77.4%) or targeted therapies (29.2%) or immunotherapy (13.4%) at Stage IV disease (67.6%).1 Common cancer types included gastrointestinal (36.6%), lung (20.8%), breast (13.6%), and hepatobiliary (10.0%) cancers.1
Based on univariate and multivariate analyses, ten independent predictors were included in the final model, which classified patients into three risk groups: low risk (0-3 points, with TRT incidence of 35.3% in the development cohort and 29.0% in the validation cohort), intermediate risk (4-8 points, with incidences of 59.7% and 61.7%), and high risk (9-26 points, with rates of 82.8% and 77.3%, respectively).1 The AUC was 0.718 (95% CI: 0.667-0.769) for the development cohort and 0.717 (95% CI: 0.616-0.818) for the validation cohort.1 Severe TRT rates differed significantly between groups, with 36.0% (36/100), 63.9% (131/205), and 86.3% (82/95) in the low, intermediate, and high-risk groups, respectively (p<0.001).1
In conclusion, this local study developed and validated a TRT prediction tool for older cancer patients receiving systemic treatment, incorporating patient characteristics, treatment factors, geriatric assessment results, and laboratory data.1 The model offers clinicians a practical tool to estimate TRT risk, supporting treatment decision-making in older adults with cancer.1
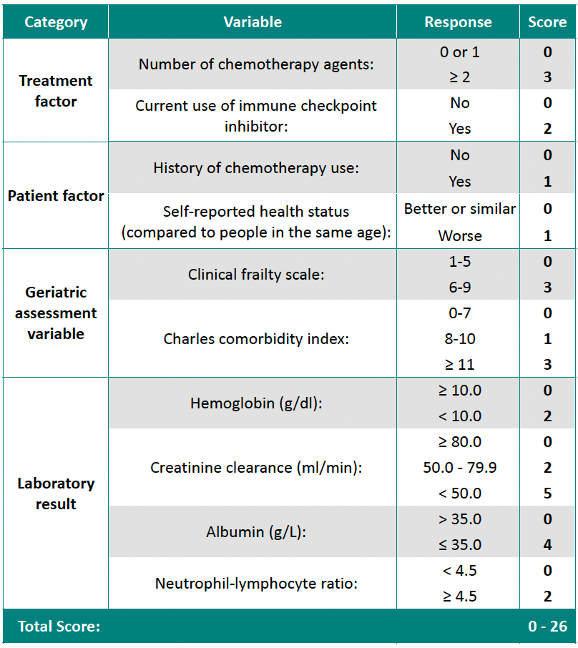
Table 1. Score calculator

