EXPERT INSIGHT
Navigating the CAR-T frontier in hematological malignancies in Asia
Chimeric antigen receptor T-cell (CAR-T) therapy has become a groundbreaking treatment for hematologic malignancies, particularly in relapsed or refractory diffuse large B-cell lymphoma (R/R DLBCL) and acute lymphoblastic leukemia (ALL).1 Building on its success in these indications, CAR-T therapy is now emerging as a promising option for patients with relapsed or refractory multiple myeloma (R/R MM), offering new hope for those with limited treatment options.1 However, the challenge of introducing CAR-T therapy into healthcare systems in Asia, is multifaceted.2,3 In an interview with Omnihealth Practice, Dr. Sivakumar Palaniappan, a consultant hematologist from Pantai Hospital Kuala Lumpur, Malaysia shed light on the region’s diverse healthcare landscape and the need for careful consideration of demographic, economic, and cultural factors to ensure broad and equitable access to this innovative treatment.
CAR-T in action: Transforming treatment for DLBCL and ALL
CAR-T therapy has demonstrated substantial promise in treating relapsed or refractory (R /R) B-cell malignancies, particularly DLBCL and ALL.1 These cancers are prevalent in Asia, while refractory cases are typically associated with poor prognosis, CAR-T therapy has significantly impacted patient outcomes, providing an option for individuals who have not responded to conventional therapies.1,4-6 In pivotal trials such as the JULIET trial (for DLBCL) and the ELIANA trial (for ALL), CAR-T therapies have shown remarkable efficacy.7,8 The JULIET trial demonstrated that tisagenlecleucel, a CAR-T therapy targeting CD19, achieved an overall response rate (ORR) of 52%, with a complete response (CR) rate of 40% in patients with R/R DLBCL.7 Similarly, the ELIANA trial showed that tisagenlecleucel was highly effective in pediatric and young adult patients with R/R B-cell ALL, achieving a CR rate of 81%.8 These results highlight the transformative role of CAR-T in hematologic malignancies, particularly for patients with limited treatment options.1
The rising potential of CAR-T in multiple myeloma
The application of CAR-T therapy has extended beyond B-cell DLBCL and ALL, with emerging evidence suggesting a promising role in the treatment of multiple myeloma.1,9 Multiple myeloma, a plasma cell malignancy, remains a challenging disease, especially in the R/R setting.1 Despite the development of newer therapies, many patients experience disease progression, making CAR-T an appealing option for this population.9
Recent pivotal trials, such as CARTITUDE-1 and KarMMa, have shown compelling results for CAR-T therapies targeting B-cell maturation antigen (BCMA) in multiple myeloma.10,11 The CARTITUDE-1 trial demonstrated that ciltacabtagene autoleucel (cilta-cel), a BCMA-targeting CAR-T, resulted in an ORR of 97% in heavily pre-treated patients, with a CR rate of 67%.10 Similarly, the KarMMa trial reported an ORR of 73% for patients treated with idecabtagene vicleucel (ide-cel), another BCMA-targeted CAR-T therapy.11
These promising results highlight the significant role CAR-T therapy has played in revolutionizing treatment options for hematologic malignancies.1,9 However, as with all advanced therapies, patient selection is critical to achieving optimal outcomes and minimizing risks. Understanding which patients are most likely to benefit from CAR-T therapy is essential in maximizing its therapeutic potential.2,6
Patient selection is key for CAR-T therapy
The key to successful CAR-T therapy lies in appropriate patient selection.2,6 Eligible patients typically include those with R/R setting who have exhausted other therapeutic options.6,9 This includes patients who have undergone at least three prior treatments or are refractory to their last line of therapy.6,9 However, for CAR-T therapy to be effective, patients must meet certain health criteria, including adequate organ function and a manageable performance status.6,9,12
Drawing from his practice in Malaysia, the selection process is influenced by the local demographic profile, which includes a significant portion of older patients and those with prevalent comorbidities such as diabetes, hypertension, and cardiovascular disease. These factors complicate the assessment of eligibility, as comorbid conditions can impact the ability to tolerate CAR-T therapy.9 As such, comprehensive management of these chronic conditions is often necessary—highlighting the broader challenge faced by the healthcare system in addressing multimorbidity.13
Furthermore, the nation’s public healthcare system, which is designed to serve a broad population, faces the challenge of balancing patient eligibility with available resources.2,14 As Dr. Sivakumar pointed out, “While the therapy holds significant promise, we must ensure that the cost-to-benefit ratio is carefully considered. We can’t just treat everyone, we need to prioritize those most likely to respond and those who would gain the most from this transformative treatment.”
Challenges of accessibility and healthcare infrastructure
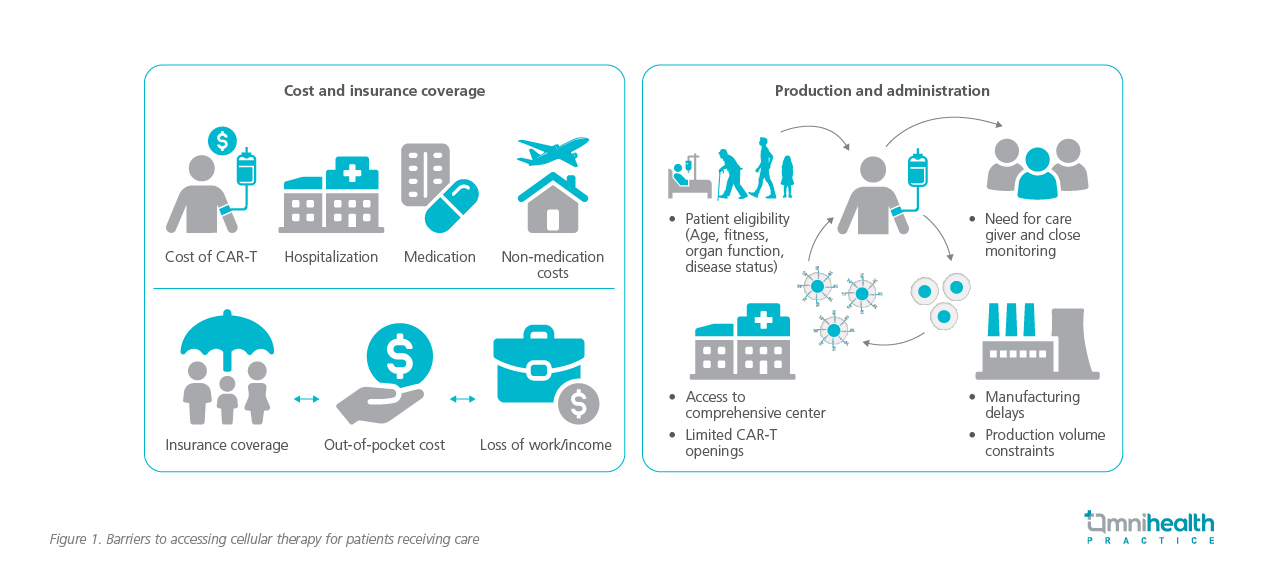
Dr. Sivakumar noted that in many Asian localities, the accessibility of CAR-T therapy is shaped by the urban-rural divide. Major urban centers typically have the necessary infrastructure to support the complex needs of CAR-T therapy, including specialized medical teams and cell-processing laboratories.15 However, rural areas, where healthcare resources are more limited, may face significant barriers in accessing such advanced treatments.2,15,16 This creates a gap in healthcare equity, as patients in underserved regions are less likely to benefit from CAR-T therapy.2,15,16
Moreover, the introduction of CAR-T therapy into a healthcare system that is still developing its capabilities for gene and cell-based therapies requires a robust infrastructure.12 Dr. Sivakumar highlights, “We must not only invest in specialized centers for CAR-T treatment but also ensure that healthcare professionals—from oncologists to nurses— are well-equipped to manage the complexities of these therapies. It’s not just about having the technology but also about having the expertise to use it effectively.”
Dr. Sivakumar further highlighted the applicability of CAR-T in a broader Asian context (figure 1):1
- Age demographics: In many Asian countries, including Malaysia, the average age of myeloma patients is younger, which could make CAR-T therapy more applicable to these populations due to better overall health
- Genetic diversity: Genetic factors, such as mutations more common in specific Asian populations, could impact how patients respond to CAR-T, requiring more localized studies
- Healthcare access and equity: The gap in healthcare access between urban and rural areas in rapidly developing Asian nations, like India, China, and Indonesia, is highlighted as a key barrier to CAR-T therapy adoption
- Cost and healthcare systems: While countries like Singapore and South Korea have the infrastructure for CAR-T adoption, others with less developed systems might struggle to afford or implement these treatments
Collaborative efforts and future perspectives
Looking ahead, the successful integration of CAR-T therapy into Asia’s healthcare systems will depend on several factors: regulatory adaptations, infrastructure development, cost management, and regional research collaborations.3,12 In particular, local clinical trials will be essential for understanding how CAR-T therapies perform in Asian populations.17 This will not only refine patient selection criteria but also optimize treatment regimens to suit local genetic profiles.2
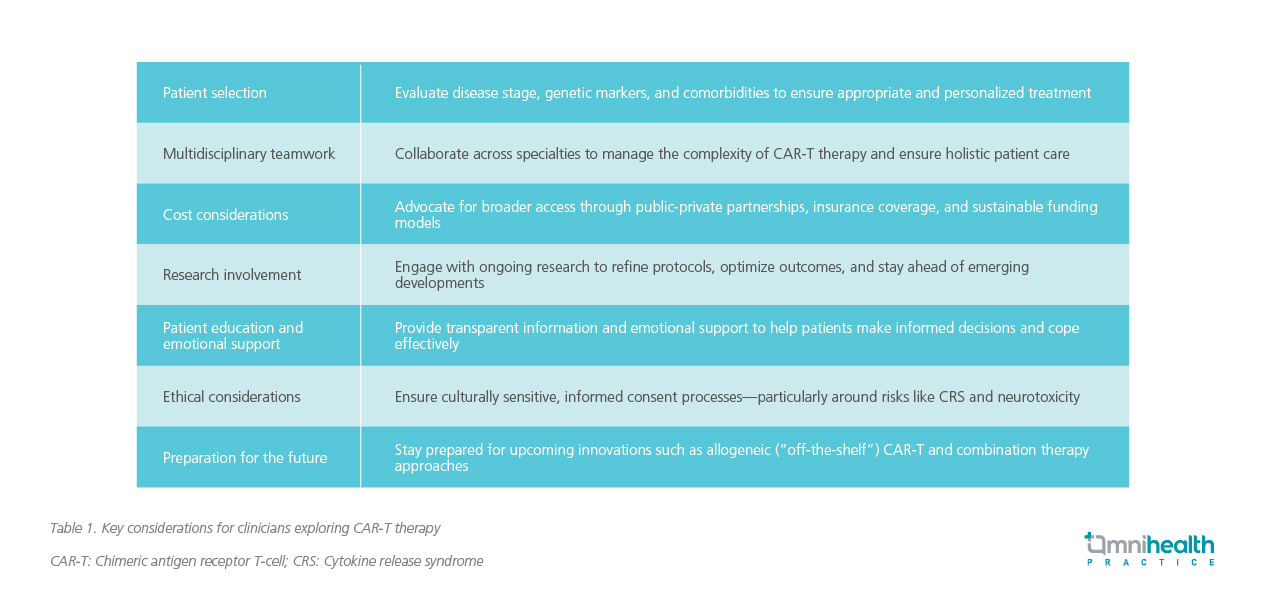
Dr. Sivakumar also shared key guidance for clinicians and budding hematologists who are interested in integrating CAR-T therapy into their practice. His insights reflect both current clinical realities and forward-looking strategies essential for successful implementation (table 1).
Conclusion
The successful integration of CAR-T therapy for hematological malignancies into Asian healthcare systems hinges on addressing patient selection, infrastructure needs, financial barriers, and cultural nuances.2-4,18 By strengthening local regulatory frameworks, improving healthcare access, and fostering regional research, the region can ensure that CAR-T therapy reaches the patients who need it most.9,19 The future of CAR-T therapy in Asia offers both significant oppor tunities and challenges, but with a coordinated and strategic approach, it can be a transformative treatment modality for hematologic malignancies.12,19