CONFERENCE UPDATE
Long-term clopidogrel monotherapy superior to aspirin in high ischemic risk patients post-PCI: Results from the SMART-CHOICE 3 trial
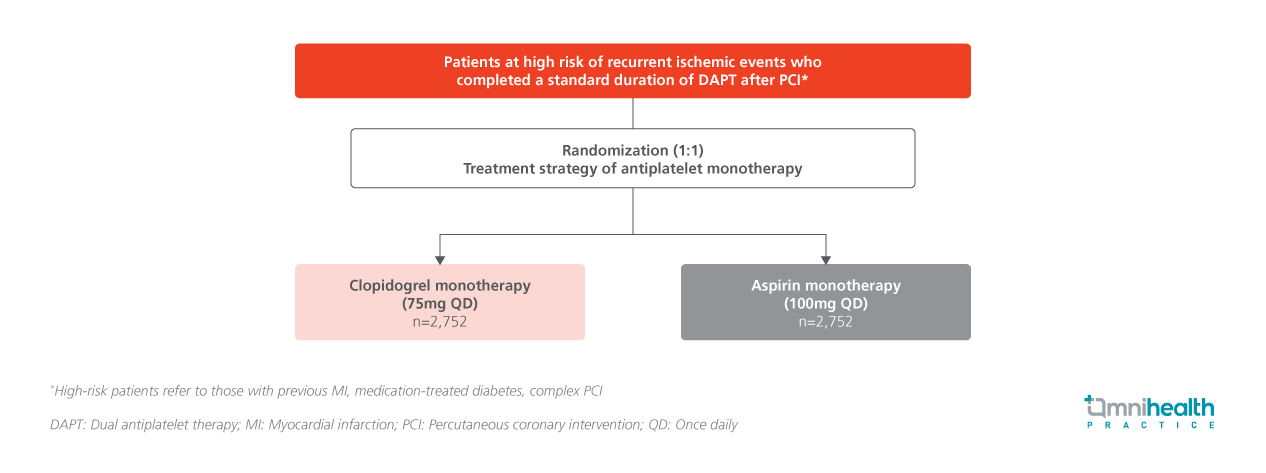
STUDY DESIGN
Indefinite aspirin monotherapy has long been the standard after dual antiplatelet therapy (DAPT) in post-percutaneous coronary intervention (PCI) patients, but its benefits remain debated.1 Emerging but limited evidence suggests that clopidogrel may be a superior alternative, highlighting the need for large-scale trials focused on high-risk populations to establish definitive guidance.1 The SMART-CHOICE 3 trial was an investigator-initiated, randomized, open-label, multicenter study conducted across 26 sites in South Korea to compare the efficacy and safety of clopidogrel vs. aspirin monotherapy beyond the standard duration of DAPT in post-PCI patients at high risk of recurrent ischemic events.1
In the SMART-CHOICE 3 trial, a total of 5,506 patients (median age: 65 years) were randomized in a 1:1 ratio to receive either clopidogrel 75mg once daily (n=2,752) or aspirin 100mg once daily (n=2,754).1 Eligible participants were adults aged 19 years or older who had undergone successful PCI with drug-eluting stents (DES) and had completed a standard duration of DAPT—defined as at least 12 months following myocardial infarction (MI) or at least 6 months after PCI for other indications.1 To qualify as high risk for recurrent ischemic events, patients were required to have at least one clinical characteristic, such as a history of MI, medication-treated diabetes mellitus, or complex coronary artery lesions.1 The median interval between PCI and randomization was 17.5 months, and the median follow-up duration was 2.3 years.1 Baseline demographics and clinical characteristics were well-balanced between the two treatment groups.1
The primary endpoint was the cumulative incidence of major adverse cardiac and cerebrovascular events (MACCE), defined as a composite of all-cause death, MI, or stroke, evaluated over the study follow-up period.1 Secondary endpoints included the individual components of the primary endpoint— MI and stroke—as well as death from cardiovascular and non-cardiovascular causes, and bleeding events classified according to the Bleeding Academic Research Consortium (BARC) criteria.1 A composite endpoint known as net adverse clinical events (NACE), comprising MACCE plus BARC type 3 or 5 bleeding, was also assessed to evaluate the overall clinical benefit and safety of each treatment strategy.
FINDINGS
| Primary endpoint: |
|
|
| Secondary endpoints: |
|
|
|
|
|
|
| Safety: |
|
"The SMART-CHOICE 3 trial was the first to demonstrate the benefits of clopidogrel monotherapy compared with aspirin monotherapy on a composite of hard endpoints in patients at a high risk of recurrent ischemic events after PCI
Dr. Joo-Yong Hahn
Samsung Medical Centre,
Seoul, Korea
Asprin may lower the risk for several gastrointestinal and colorectal cancer, reports up-to-date meta-analysis
An up-to-date meta-analysis investigated the relationship between regular aspirin consumption and cancers of the digestive tract sites. The review included 113 observational studies published up to March 2019, containing 45 colorectal

