EXPERT INSIGHT
Simplifying care: Alleviating treatment burden in APL with oral arsenic trioxide
Acute promyelocytic leukemia (APL) has historically been one of the most aggressive forms of leukemia and an oncological emergency, characterized by rapid progression and high early mortality rates.1 Traditional chemotherapy treatments often led to dismal prognoses, but the discovery of targeted therapies such as all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) has revolutionized its management.1 The ongoing evolution of ATO formulations is highlighted by an Acute Promyelocytic Leukaemia Asian Consortium (APL-AC) study, which examines the efficacy and safety of oral-ATO. This study underscores the potential of oral-ATO not only to enhance patient outcomes in APL but also to provide a more convenient and cost-effective option.2 In an interview with Omnihealth Practice, Dr. Gill Harinder Singh Harry, the study’s lead investigator, described how the development of an oral formulation of ATO has further transformed the treatment landscape, offering significant improvements in survival rates, quality of life (QoL), and healthcare resource utilization.
Historical hurdles in APL and need for alternative treatment options
APL is a subtype of acute myeloid leukemia (AML) marked by the abnormal accumulation of promyelocytes in the bone marrow and blood.1 APL has historically been regarded as one of the most aggressive types of leukemia, with high early mortality rates resulting from its rapid progression and complications associated with standard chemotherapy treatments.1 Targeted therapies like ATRA and ATO have shifted treatment away from conventional chemotherapy and greatly improved survival rates and safety profiles.3 This evolution towards personalized treatment enhances patient outcomes in APL management and also shows promise for other hematological disorders.4
Despite these advancements, Dr. Gill stated that despite these immense improvements over chemotherapy, intravenous (IV) administration of ATO still leads to considerable inconvenience and high healthcare costs. Recognizing the potential benefits of an alternative formulation, Dr. Gill and his team advocated for an oral formulation and spearheaded the APL-AC study to assess its efficacy.2 The study evaluated the impact of ATRA/chemotherapy (n=436), ATRA/IV-ATO (n=61), and a regimen of oral-ATO/ATRA/ascorbic acid (oral-AAA) with ATO maintenance (n=150) on survival and relapse rates across a wide range of APL patients who were followed for over 2 decades.2 The cohort comprised an almost even distribution of genders, with a median age of 45.5 years, indicating a diverse demographic range (18.1-91.8 years).2 Patients with low-/intermediate-risk (n=448) and high-risk (n=199) diseases were included.2
Improved survival outcomes with oral-ATO and IV-ATO regimens
The study showed that both oral-ATO and IV-ATO regimens significantly improved survival outcomes, including reduced early deaths (within 60 days), improved overall survival (OS), and higher 5-year relapse-free survival (RFS). 2 The ATRA/chemotherapy group had significantly more early deaths within 60 days compared with the ATO groups (8.3% vs. 3.3%; p=0.05) (figure 1A).2 The results extended beyond the 60 days, with OS rates of 84.6% for ATRA/ chemotherapy, 91.4% for ATRA/ IV-ATO, and 92.3% for oral-AAA (p<0.03) (figure 1B).2 Additionally, the 5-year RFS rates were 76.9% for ATRA/chemotherapy, compared to 92.8% for ATRA/IV-ATO and 97.8% for oral-AAA (p<0.001) (figure 1C).2
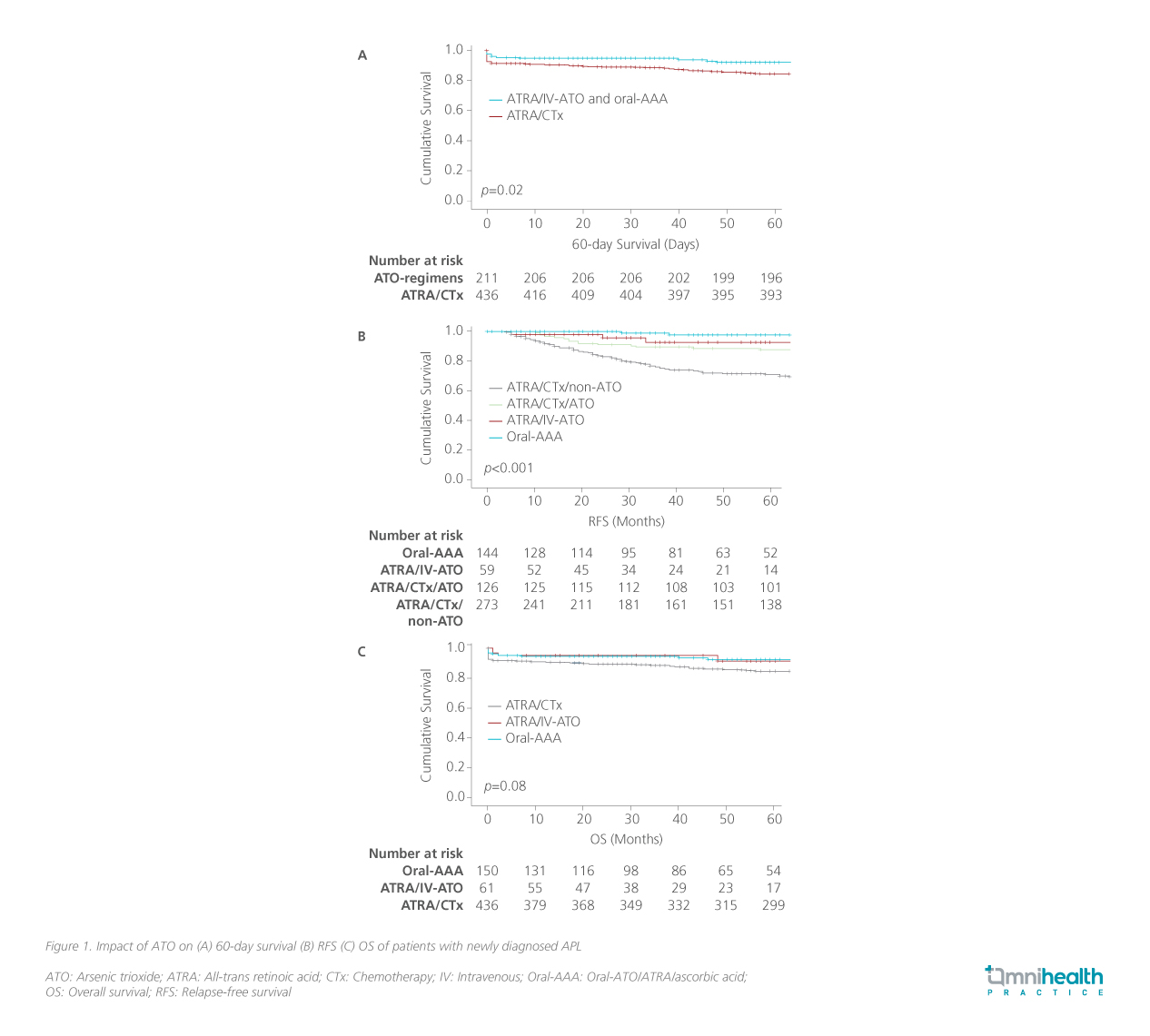
Survival data stratified by patient group
Further analysis of the results has shown that the benefit was sustained across patients with different severities of APL.2 It was revealed that better survival outcomes were seen in patients receiving ATO-inclusive regimens across all risk categories.2 When ATO was included in the treatment regimen—whether during induction, consolidation, or maintenance—the survival rates of high-risk patients matched those of the low-/intermediate-risk group, regardless of the formulation used.2 Conventional risk factors relevant to patients receiving chemotherapy such as presentation of leukocyte and platelet counts were no longer prognostically relevant in patients receiving oral-ATO.2
Beyond risk factors, Dr. Gill emphasized the promising results observed across both the newly diagnosed cohort and the relapsed cohort. In the newly diagnosed cohort, the relapse rate was extremely low, with most patients passing away primarily due to advanced age instead of the disease, and no patients discontinued treatment due to safety concerns or withdrew for any reason. In relapsed APL patients, Dr. Gill maintained that reinduction with oral-ATO retains a high five-year OS rate without any treatment-related mortality. This eliminates the need for bone marrow transplant (BMT), which is currently the standard of care despite its high treatment mortality risk.
Treatment advantages beyond survival and relapse
In addition to survival outcomes, oral-ATO reveals more advantages, particularly in terms of patient convenience and healthcare resource utilization.4 Oral-ATO allows for easier administration at home, which is a major shift from the traditional IV-ATO regimen that necessitates frequent hospital visits and IV access.4 This transition not only reduces the need for hospitalization but also streamlines the treatment process, leading to fewer hospital visits and lower staff involvement.4 As a result, the burden on healthcare facilities is significantly diminished, enhancing operational efficiency and substantially lowering overall treatment costs.4
Beyond these logistical benefits, the affordability of oral- ATO further enhances its attractiveness, particularly for the long-term management of APL.4 The cost-effectiveness of oral-ATO is critical, especially in regions like Asia, where healthcare resources are often limited and financial constraints can impede access to essential treatments.4 The availability of an affordable oral option can be transformative, enabling a broader patient population to receive effective treatment without the prohibitive costs associated with hospital-based therapies.4
Moreover, the benefits of oral-ATO extend beyond convenience and economic considerations.4 By allowing patients to take their medication at home, oral-ATO significantly improves QoL.4 Patients experience less disruption to their daily routines, which is vital for long-term care.4 This ease of administration is associated with better adherence to treatment protocols, leading to enhanced OS rates and a notable increase in the prevalence of APL survivorship.4 These improvements in patient outcomes reflect advancements in clinical practices and signify a broader positive socioeconomic impact.4
By addressing critical issues of accessibility and affordability in the treatment landscape for APL, oral-ATO represents a paradigm shift that aligns with the evolving needs of patients and healthcare systems.4 The transition underscores the importance of providing patients with effective, accessible, and convenient treatment options, ultimately fostering a healthcare environment that prioritizes patient-centered care and equitable access to life-saving therapies.4
Prioritizing global accessibility and adoption of oral-ATO
To optimize the global practice of treating APL with oral-ATO, it is imperative to prioritize the accessibility and adoption of oral-ATO formulations, particularly in lower-income regions where healthcare resources are limited. Dr. Gill highlighted that oral-ATO has been classified as an investigational new drug (IND) by the Food and Drug Administration (FDA), meeting the prerequisites for phase 3 registrational studies worldwide. While IV-APO is not reimbursed in many areas, oral-ATO has a much higher likelihood of receiving reimbursement with the growing evidence from both global and local trials. Dr. Gill also remarked that despite the clear advantages that oral-ATO offers, there remains a significant reliance on IV-ATO in many parts of the world. This continued dependency not only leads to increased healthcare costs but also results in unnecessary patient suffering and preventable deaths, as many individuals may not have the resources or capability to access regular hospital treatments. To address this disparity, stakeholders—including healthcare providers, policymakers, and pharmaceutical companies—must work collaboratively to develop strategies that facilitate the widespread adoption of oral-ATO.
Dr. Gill also mentioned that drug repurposing studies on oral-ATO in other subtypes of AML and autoimmune diseases are currently underway, as it has implications in suppressing TP53, inflammation, and T lymphocytes. ATO holds significant potential as a therapeutic agent for a variety of non-APL diseases, particularly autoimmune disorders and idiopathic pulmonary fibrosis (IPF), both of which have a higher prevalence than APL.4 Preliminary studies indicate that oral-ATO could exert beneficial effects on these conditions, providing a novel treatment option for patients who currently face limited choices.4 The established safety profile of oral-ATO further enhances its appeal, making it a compelling candidate for future research aimed at exploring its efficacy in these diverse diseases.4 As clinical investigations expand to include non-APL indications, the adaptability of oral-ATO may not only contribute to improved patient outcomes across a broader spectrum of disorders but also reinforce the substance's value in clinical practice.4 By validating its therapeutic roles beyond APL, oral-ATO could become a cornerstone treatment for many chronic conditions, significantly impacting the QoL for countless patients battling these challenging ailments.4
Conclusion
The introduction of oral-ATO has significantly transformed the treatment landscape for APL, markedly improving survival rates as demonstrated by the APL-AC study.2 Reflecting on the transformative potential of oral-ATO in modern medicine, there is hope for enhanced patient outcomes and improved QoL globally, reinforcing the commitment to equitable healthcare access and innovative therapies for all.4 Its promising implications extend beyond APL, suggesting potential therapeutic benefits for various other diseases, signaling a need for increased research and clinical trials in these areas.4 Dr. Gill reiterated that there is a call to action for advocating wider accessibilit y of oral-ATO, particularly in resource-limited settings where patients face substantial barriers to ef fective treatment.

