CONFERENCE UPDATE: CROI 2025
Zero HIV acquisition and high persistence with CAB LA as PrEP: 12-month results from the PILLAR trial
STUDY DESIGN
In 2022, men who have sex with men (MSM) and transgender men (TGM) accounted for 67% and <1% of new HIV diagnoses in the United States, respectively.1 Additionally, an estimated 15% of MSM and 3% of TGM were living with HIV.1 Long-acting cabotegravir (CAB LA), administered every two months via intramuscular injection, is the first and only approved long-acting medication for human immunodeficiency virus 1 (HIV-1) pre-exposure prophylaxis (PrEP) in adults and adolescents.1 It has demonstrated superiority to daily oral PrEP (tenofovir disoproxil fumarate + emtricitabine) in preventing new HIV acquisition.1 CAB LA has consistently shown high effectiveness and tolerability across both clinical trials and real-world implementation studies.1
The PILLAR trial evaluates the real-world adoption and clinical outcomes of CAB LA, with this analysis presenting 12-month findings on HIV incidence, persistence, and safety.1 The PILLAR trial is a phase 4, real-world, implementation science study designed to assess CAB LA integration into clinical practice.1 In this site-randomized study, 17 sites were assigned to either dynamic CAB LA implementation (n=11) or routine CAB LA implementation (n=6).1 A total of 201 participants were enrolled and initiated CAB LA, with a median age of 35 years (interquartile range [IQR]: 29-44); 6% were TGM, 26% were Black, and 38% were Hispanic.1 Notably, 22% had not used oral PrEP in the six months prior to CAB LA initiation.1 Participants were eligible to initiate CAB LA through either a direct-to-inject approach or an oral lead-in phase, followed by every-2-month (Q2M) CAB LA injections.1 Over a 12-month follow-up period, the study assessed key endpoints including HIV incidence, HIV testing, persistence (continuation of CAB LA use), safety and tolerability.1
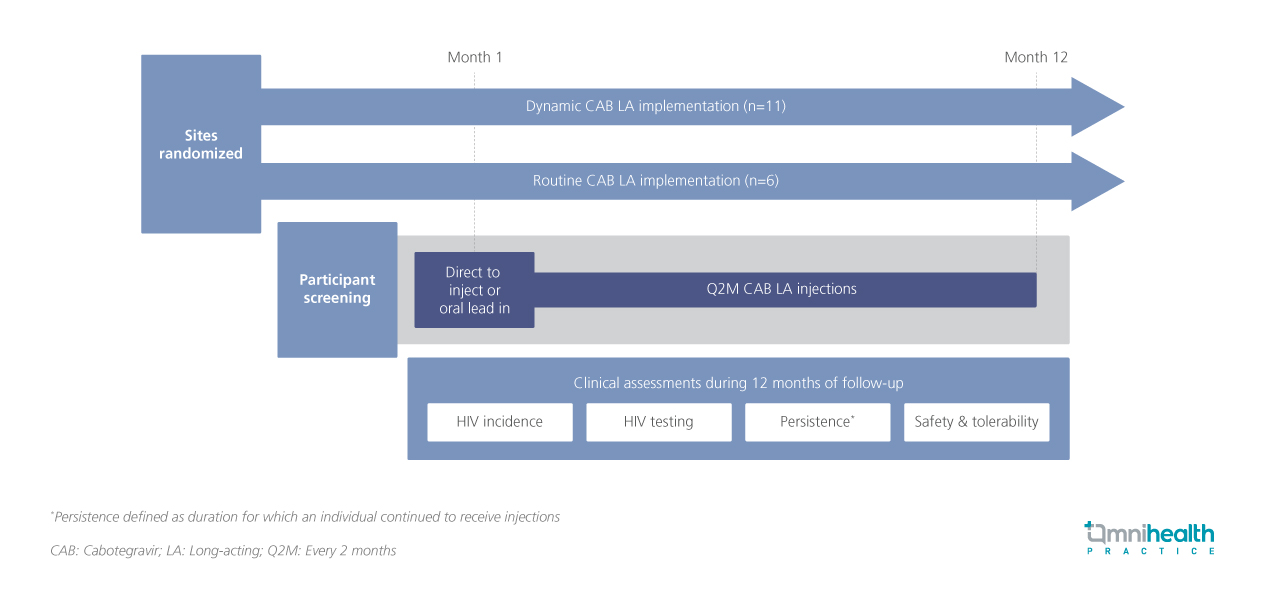
FINDINGS
|
Key endpoints: |
|
|
|
|
|
|
|
| Safety: |
|
|
"Zero cases of HIV acquisition were found through month 12, supporting the robust and sustained effectiveness of CAB LA in the real-world setting
Dr. Taimur Khan
Fenway Health, Boston,
United States

