CASE REVIEW
Revolutionizing psoriasis treatment: Local experience with deucravacitinib in difficult-to-treat areas
Psoriasis (PsO) is a chronic inflammatory skin disease marked by persistent inflammation and abnormal differentiation of keratinocytes.1 In difficult-to-treat areas such as the scalp and palms, treatment options commonly used to treat PsO may deliver a weaker response.1 Deucravacitinib, a first-in-class oral tyrosine kinase 2 (TYK2) inhibitor, has emerged as a promising therapeutic option for moderate-to-severe plaque PsO, particularly in patients with difficult-to-treat areas.2 This article discusses a case experience of PsO management with deucravacitinib, emphasizing the critical factors influencing therapy selection, including the patient's specific symptoms, previous treatment responses, and concerns about side effects. Additionally, data from clinical trials demonstrate the efficacy and safety of deucravacitinib, reinforcing its potential as a valuable addition to the PsO treatment landscape.
Background
PsO is an immune-mediated inflammatory skin disease characterized by persistent inflammation, which leads to abnormal proliferation and differentiation of keratinocytes.1 Despite available treatments, outcomes for PsO remain suboptimal, with many patients feeling under-treated or dissatisfied with the effectiveness and side effects of conventional therapies, particularly in difficult-to-treat areas.3 A step ladder approach is recommended for PsO treatment, starting with topical treatments for most mild to moderate cases, while approximately 20% of patients with moderate-to-severe PsO may require more intensive interventions. It is crucial for treatment to align with PsO severity and individual health profiles of patients, as many patients are undertreated with ineffective topical options and would benefit from safer oral treatment.
Deucravacitinib is a first-in-class, oral, selective, allosteric TYK2 inhibitor approved for the treatment of adults with moderate-to-severe plaque PsO who qualify for systemic therapy.4 It uniquely binds to the regulatory domain of TYK2.4-6 By selectively inhibiting TYK2, an intracellular enzyme involved in the pathogenesis of PsO, deucravacitinib modulates the signaling of key inflammatory cytokines involved in PsO (e.g. IL-12, IL-23 and type I interferons) without broadly inhibiting other JAK pathways, which could lead to fewer side effects.4,7 During in vitro studies, deucravacitinib has shown 100- to 2,000-fold selectivity for TYK2 compared to JAK1, JAK2, and JAK3.7 Deucravacitinib represents a promising option for managing moderate-to-severe PsO, particularly in patients with involvement in difficult-to-treat areas such as the scalp and palmoplantar PsO and is suitable for patients with comorbidities or those who prefer oral treatment over injections.8-10 To demonstrate the efficacy of deucravacitinib in PsO with difficult-to-treat areas, a case experience has been presented.
Case sharing
A 65-year-old female presented with a year-long history of an itchy rash primarily affecting her palms and soles, which worsened after bathing and significantly disrupted her sleep. She also exhibited subungual hyperkeratosis on her fingernails and toes. Her medical history included elevated lipids, for which she was on a statin, and mild eczema on her upper back.
During her examination in the first consultation, she was found to have PsO, more pronounced on the right side, along with thick, red, dry, and cracked skin on her extremities. Pre-screening tests, including a tuberculosis (TB) Gold test and chest X-ray, returned normal results. Previous treatments with ketoconazole cream and clobetasol/betamethasone ointments had yielded only partial relief, prompting further evaluation and management.
During the initial consultation, various treatment options were discussed, including pine tar, oral retinoids, methotrexate, and deucravacitinib. The patient had concerns about the side effects associated with systemic therapies like methotrexate. Given the localized nature of the patient’s PsO and her suitability, the decision was made to initiate her on deucravacitinib, with a follow-up appointment scheduled for 4 weeks later.
Prior deucravacitinib initiation, the patient experienced anxiety regarding potential side effects due to her previous experiences with oral retinoids. Initially, her symptoms worsened, and she developed new itchy rashes in the genital area. However, blood tests indicated no significant abnormalities. After 4 weeks of treatment, notable improvements began to surface; the discomfort in her genital area subsided, and there was a slight improvement in her hand and foot symptoms, with reduced itching.
Significant clinical improvements were observed by 8 weeks: there was a marked reduction in redness and keratotic lesions, and the patient reported better sleep due to decreased itching. Minor skin thickening remained, but an overall improvement in her skin's appearance and texture (an 80% improvement in symptoms on her hands and a 70% improvement on her feet [figure 1 and 2]), contributing to her overall better state of health. Routine monitoring involved regular liver function tests, complete blood counts, and assessments for infection risks. As the patient had pre-existing hypercholesterolemia prior to treatment, checks on her lipid levels were also conducted.
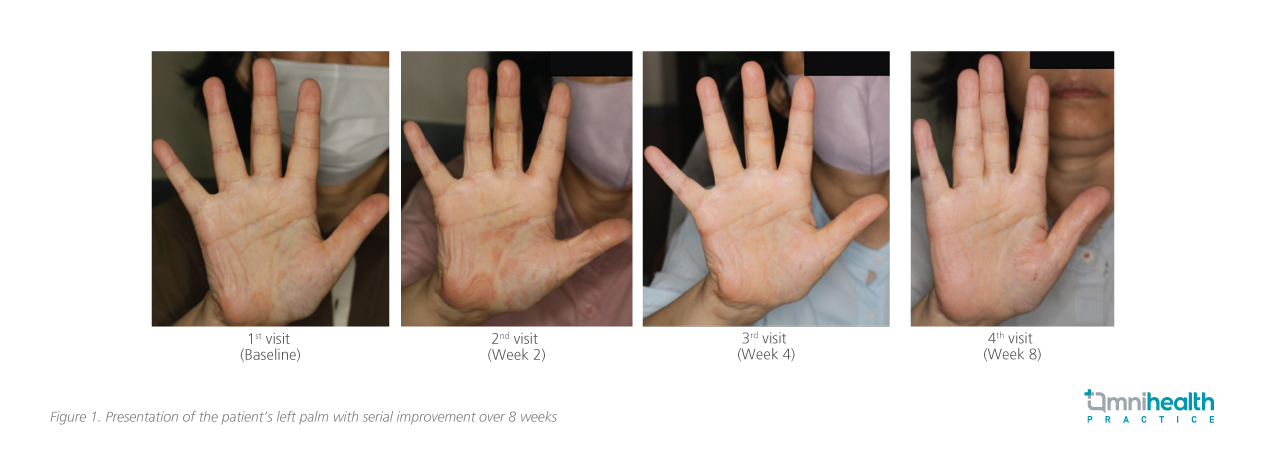
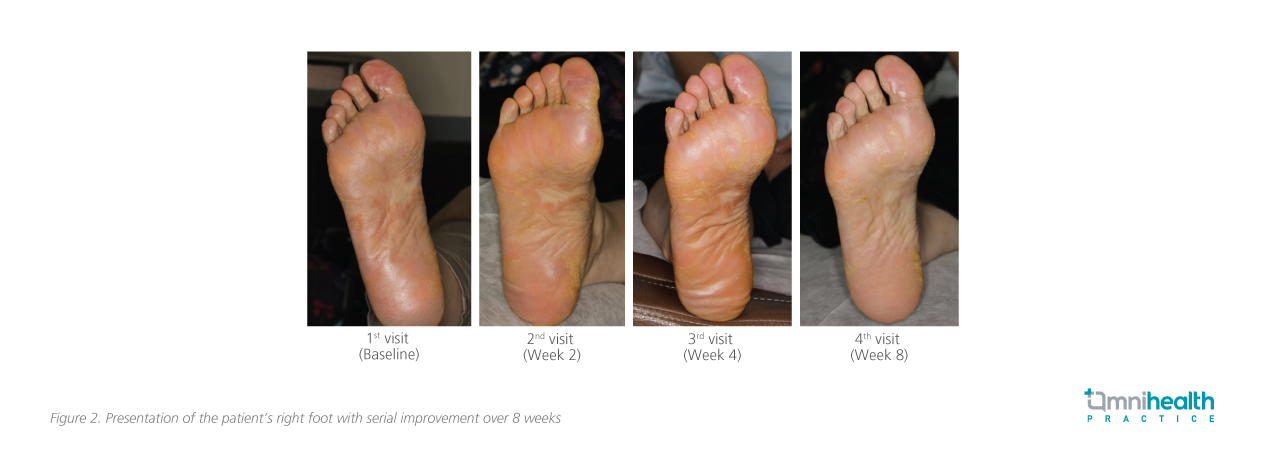
The patient expressed high satisfaction with her treatment, noting that her primary concerns were effectively addressed. She achieved her treatment goals and was eager to continue monitoring her condition while maintaining her routine, including receiving annual flu vaccinations.
Overall, deucravacitinib demonstrated significant efficacy in managing moderate-to-severe PsO in this patient, with a favorable safety profile and improved quality of life. This case highlights the drug's potential as a cost-effective alternative to biologics, particularly for patients with localized disease and concerns regarding side effects from traditional systemic therapies.
Discussion
Consistent efficacy of deucravacitinib demonstrated in clinical trials The efficacy of deucravacitinib shown in the case presented aligns with data from global phase 3 studies.2,9,10 The POETYK PSO-1 and POETYK PSO-2 trials established its efficacy and safety in patients with moderate-to-severe plaque psoriasis, supported by long-term follow-up.2,9,10
In the phase 3 randomized POETYK PSO-1 trial, patients with moderate-to-severe PsO (defined as static Physician's Global Assessment [sPGA] ≥3, Psoriasis Area and Severity Index [PASI] ≥12, and body surface area [BSA] involvement ≥10% for ≥6 months before screening) were randomized to receive deucravacitinib 6mg (n=332), apremilast (n=166) or placebo (n=168) for 52 weeks.2 Compared with placebo, deucravacitinib led to a significantly higher rate of ≥75% reduction from baseline in PASI (PASI 75), (58.4% vs. 12.7%; p<0.0001), ≥90% reduction from baseline in PASI (PASI 90), and sPGA of 0 or 1 (sPGA 0/1) (53.6% vs. 7.2%) at week 16, establishing its potential in minimizing disease activity and symptom improvement.2
Longer-term use of deucravacitinib 6mg daily was investigated in the POETYK open-label long-term extension (LTE) conducted in patients who had ≥1 dose of deucravacitinib (n=1,519) in POETYK PSO-1 and POETYK PSO-2.11 Its efficacy was maintained at 4 years with PASI 75 retained throughout the LTE trial.11
Targeting difficult-to-treat areas with deucravacitinib
In the pooled analyses of the POETYK PSO-1 and PSO-2 trials, deucravacitinib was also shown to be effective against moderate-to-severe PsO in difficult-to-treat areas defined by a score of ≥3 on the scalp-specific Physician's Global Assessment (ss-PGA), PGA-Fingernails (PGA-F), and palmoplantar PGA (pp-PGA) scores for scalp, fingernail, and palmoplantar PsO respectively.10 The analysis found that deucravacitinib consistently relieved symptoms in these specific areas.10 At week 16, patients receiving deucravacitinib demonstrated significantly higher response rates, notably, in achieving pp-PGA 0/1 for palmoplantar areas compared to those on placebo (49.1% vs. 16.0%; p<0.0001) (figure 3).10 The response rates for all three assessments continued to increase after patients in the placebo arm crossed over to deucravacitinib treatment following week 16.10

Overall, the data confirmed that deucravacitinib provides sustained clinical improvements in PsO symptoms, supporting its use as an effective treatment for patients with moderate-to-severe plaque PsO.
Favorable safety outcomes in long-term extension
Besides its efficacy in difficult-to-treat areas, deucravacitinib has also shown comparable safety profiles with placebo in clinical trials, which was extended to clinical practice.11 The safety outcomes of deucravacitinib from the POETYK PSO-1, PSO-2, and LTE trials indicated that the treatment was generally well-tolerated.11 Over 4 years, the most common adverse events (AEs) included nasopharyngitis (9.7 exposure-adjusted incidence rate [EAIR]/100 person-years [PY]), upper respiratory tract infections, and headache.11 Serious infections, including COVID-19, remained rare at 2.0 EAIR/100 PY through the 4 years.11 Malignancy incidence rates were low and similar at both the 1-year and 4-year marks.11 The incidence rate of malignancies (excluding nonmelanoma skin cancer) associated with deucravacitinib over 4 years was in line with those observed in other trials (0.4-0.6 EAIR/100 PY).11 Overall, serious and malignancy-related events remained low.11 Importantly, no new safety signals were identified during long-term treatment, making it a safe option for patients with moderate-to-severe plaque PsO.11 Overall, the safety profile of deucravacitinib was consistent with previous findings.11
Conclusion
In summary, this case review highlights deucravacitinib, a first-in-class oral TYK2 inhibitor, as an effective option for managing moderate-to-severe PsO, particularly in challenging areas such as the scalp and palms. The reported patient case demonstrated significant improvements, with an 80% reduction in hand symptoms and a 70% reduction in foot symptoms within 8 weeks. The clinical experiences are consistent with findings from global phase 3 trials, which indicate that deucravacitinib effectively targets key inflammatory pathways while maintaining a favorable safety profile.2,9,10 Overall, deucravacitinib may serve as a valuable addition to current treatment strategies for PsO.

