MEETING HIGHLIGHT
Reaching for greater heights with durvalumab regimens in BTC and uHCC
Durvalumab, a programmed death-ligand 1 (PD-L1) inhibitor, has emerged as a prominent treatment option in the management of biliary tract cancer (BTC) and hepatocellular carcinoma (HCC).1,2 At the symposium titled New Heights in Hepatobiliary Cancers with Immunotherapy, Dr. He, Aiwu Ruth and Professor Chan, Lam Stephen shared their insights as the 2nd author of the TOPAZ-1 study and the co-first author of the HIMALAYA study, respectively. Durvalumab was the first immuno-oncology (IO) agent approved for advanced BTC based on the TOPAZ-1 study.1,3-6 Moreover, the HIMALAYA study demonstrated the efficacy of durvalumab + tremelimumab as a first-line treatment for unresectable HCC (uHCC), marking the first IO-IO combination regimen for this indication including in patients from Hong Kong.2,5,8-10 Dr. Chiang, Chi-Leung and Dr. Chan, Long Landon supplemented the presentation with their case sharing. These landmark approvals have solidified the role of durvalumab-based regimens in HCC and BTC, highlighting the increasing prominence of immunotherapy against cancers.
Breakthrough with first IO in BTC after decade-long hiatus
The gemcitabine and cisplatin (Gem-Cis) combination has been the standard of care (SoC) for BTC since 2010 and has remained so for over a decade.4 The addition of durvalumab, an IO agent, to BTC management has been supported by the TOPAZ-1 study.1,4 Dr. He noted that “Patients initially diagnosed with unresectable BTC tend to have a greater likelihood of biliary obstruction and tend to do worse.”
TOPAZ-1 is a phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with Gem-Cis in patients with advanced BTC.4 The study recruited patients with a balanced tumor location distribution and recurrent or local diseases.4 Patients with previously untreated, locally advanced, or metastatic BTC (n=685) at initial diagnosis, experienced disease recurrence >6 months after prior curative surgery or adjuvant therapy, and had an ECOG PS (Eastern Cooperative Oncology Group-performance status) of 0 or 1 were included.4 Patients were randomized 1:1 to receive intravenous durvalumab 1,500mg or placebo combined with Gem-Cis every 3 weeks (Q3W) for up to 8 cycles.4 Gem-Cis was administered at 1,000mg/m2 and 25mg/m2 on days 1 and 8 for each cycle.4 Upon completion of Gem-Cis therapy, patients continued on durvalumab 1,500mg monotherapy or placebo Q4W until disease progression or unacceptable toxicity.4
Enduring OS in TOPAZ-1 as the basis of international validation
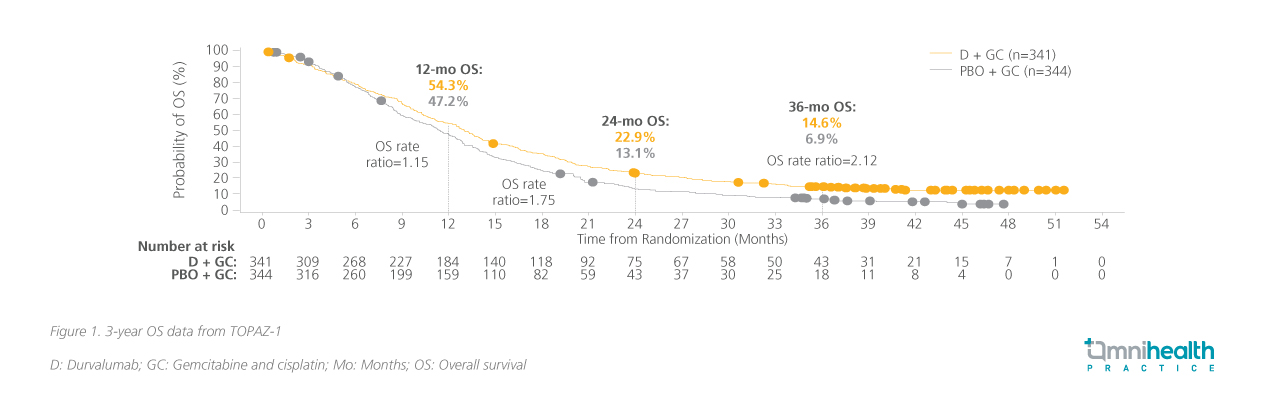
At the primary analysis, significantly greater overall survival (OS) was reached in the durvalumab arm compared to the placebo arm (hazard ratio [HR], 0.80; 95% CI: 0.66-0.97; p=0.021).4 At the current 36-month analysis, the survival benefits established in year 1 have been expanded with durvalumab + Gem-Cis and are now double that of the placebo + Gem-Cis arm (figure 1).11 Additionally, the extended long-term survival (eLTS), defined as a ≥30 months survival, was seen across various subgroups, suggesting that no single factor drove long-term survivorship.12 A higher proportion of participants were considered eLTS in the durvalumab + Gem-Cis arm (17.0%) compared to the placebo + Gem-Cis arm (8.7%).13 Among participants who responded to treatment, 1 in 3 patients in the durvalumab + Gem-Cis arm was alive for at least 30 months, compared to 1 in 4 from the placebo + Gem-Cis arm.13 Immune-mediated adverse events (imAEs) were reported at only 12.7% with durvalumab and 4.7% with placebo with the incidence of grade 3-4 imAEs at 2.4% vs. 1.5% respectively.4 Based on these updated survival and safety data, Dr. He recognized “the use of durvalumab + Gem-Cis as the SoC treatment in diverse patients with locally advanced or metastatic BTC.”
The European Society of Medical Oncology (ESMO) has evaluated the use of durvalumab in unresectable or metastatic BTC very favorably using their Magnitude of Clinical Benefit Scale (ESMO-MCBS).14 The scale considers factors such as OS, progression-free survival (PFS), disease-free survival (DFS), HR, molecular response rate, quality of life (QoL), prognosis of the condition, and toxicity.15 Under this indication, durvalumab + Gem-Cis has scored 4 out of 5 in the ESMO-MCBS, signifying its substantial treatment benefits.14,15 Dr. Chan followed up on the TOPAZ-1 data with his clinical case sharing.
Exploring real-life applications of IO in BTC
In early 2023, a 66-year-old man with BTC presented with right upper quadrant abdominal pain and jaundice. Imaging tests revealed multiple tumors in his bile ducts. A biopsy confirmed cholangiocarcinoma. Due to the extent of the disease, the patient was not initially considered a candidate for surgery. Instead, he received durvalumab + Gem-Cis. After 3 cycles, follow-up scans showed a significant reduction in the size and activity of the tumors. The enlarged lymph nodes also appeared to be non-cancerous at cycle 6. With this good response, the patient was able to undergo extensive liver surgery in 2024 to remove the remaining cancer. The surgery was successful, significantly improving his clinical prognosis. HIMALAYA: Trailblazing with the first IO-IO approach to uHCC Besides BTC, durvalumab has also proven invaluable as the first IO-IO regimen in uHCC when combined with tremelimumab.2 Anti-cytotoxic T-lymphocyte associated protein 4 (CTLA4) in combination with anti-PD-L1 agents has become an effective treatment option for uHCC, and the optimal regimen in combination with durvalumab was refined to minimize adverse effects.2,7 The HIMALAYA study is a randomized, open-label, multicenter, global phase 3 study involving uHCC patients (n=1,171) who had no prior systemic therapy, Barcelona Clinic Liver Cancer (BCLC) stage B or C, ECOG PS 0-1, and Child-Pugh score A.7 These patients were randomized 1:1:1 to receive sorafenib 400mg twice daily, durvalumab 1,500mg Q4W, or the STRIDE regimen (a single dose of tremelimumab 300mg infusion and durvalumab 1,500mg Q4W).7
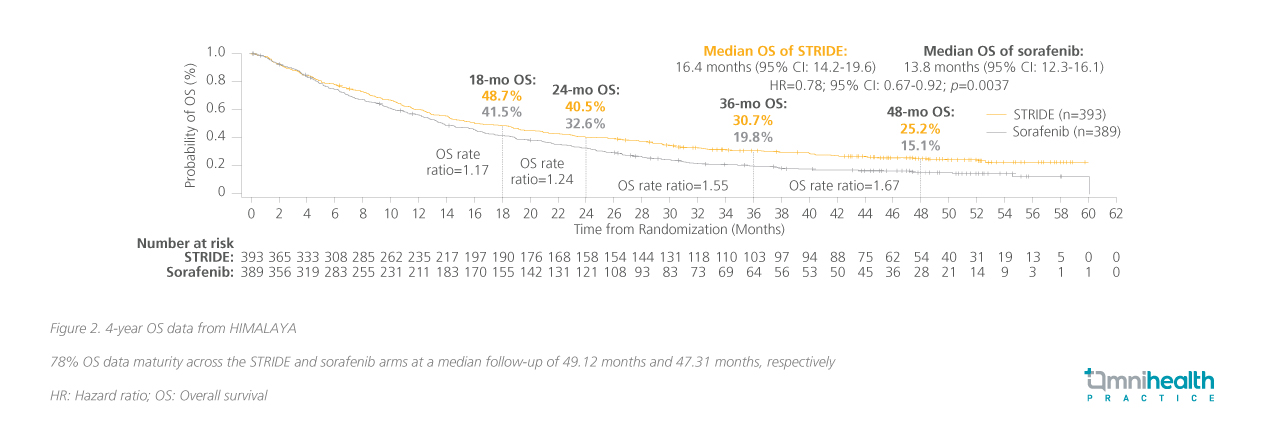
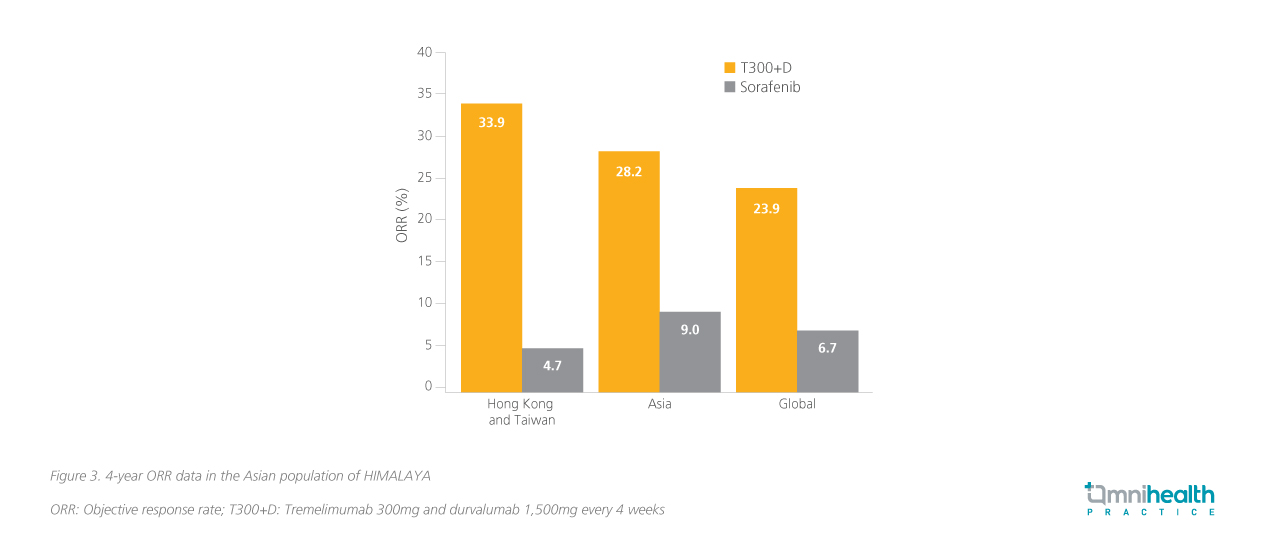
STRIDE's remarkable sustained response in both global and local populations In the primary analysis, significantly greater OS benefit had been achieved with STRIDE over the sorafenib arm (HR=0.78; 96.02% CI: 0.65-0.93; p=0.0035).7 Prof. Chan commented that, “The impression was that other therapies tend to have a very quick response, but the median time to response of IO-IO combinations, was also really impressive, as early as 2 months after treatment initiation.” Here he presented a 4-year update where the OS benefit had been maintained (HR=0.78; 95% CI: 0.67-0.92; p=0.0037) with a median OS of 16.4 months and 13.8 months with STRIDE and sorafenib, respectively (figure 2).8 Notably, a subgroup of patients in HIMALAYA came from Hong Kong and Taiwan where the risk reduction in OS was numerically greater (HR=0.44; 95% CI: 0.26-0.77).9 Prof. Chan noted that “Resistance is common after 3 years of anti-vascular endothelial growth factor therapy (VEGF) therapy. The STRIDE regimen has been shown to plateau the OS curve at 4-year and the trend is expected to continue in year 5. This is very encouraging, especially in indications such as uHCC.” The benefit of the STRIDE regimen extends beyond the primary endpoint of OS especially among the local population, also offering an ORR of 33.9% compared to just 4.7% with sorafenib (figure 3).9
Characterizing imAEs and monitoring the safety of STRIDE
Lastly, Prof. Chan discussed the safety profile from HIMALAYA after 4 years of follow-up. Overall, STRIDE was shown to be a tolerable treatment where no treatment-related gastrointestinal or esophageal varices hemorrhage events were observed during the study period.7 Incidences of treatment-related hepatic failure, fibrosis and cirrhosis were lower with the STRIDE regimen compared to sorafenib (0.8% vs. 1.6%).7 Prof. Chan highlighted that, unlike other agents, no vascular complications were reported with STRIDE, and that “You don’t need to worry about bleeding, proteinuria, or hypertension.” Furthermore, the majority of imAEs occurred within the first 3 months only.8 To demonstrate the implementation of IO-IO therapy in the clinical setting, Dr. Chiang shared a case involving the STRIDE treatment, given to a patient with HCC in his practice.
Lessons learned from IO-IO uHCC patient journey
The 83-year-old male patient had a history of hypertension, benign prostatic hyperplasia, asthma, and ischemic heart disease and was also a hepatitis B carrier on entecavir treatment. Although he was asymptomatic at presentation, elevated alpha-fetoprotein (AFP) levels level of 1,058ng/mL prompted further investigations. The patient's liver function was well-preserved (Child-Pugh A5, ALBI grade 2). Imaging studies revealed two HCC lesions (12.3cm and 2.3cm) in the liver with invasion of the hepatic veins and suspected portal vein involvement. He was offered the STRIDE regimen. After 2 cycles, AFP dropped to around 200ng/mL, and remission was achieved at cycle 6. He was placed under close surveillance and experienced a suspected solitary relapse after 17.8 months where a CT-guided RFA was performed and has since been disease-free with a good quality of life. Dr. Chiang concluded that the STRIDE regimen is “highly effective and safe in unresectable HCC and that STRIDE is promising with no new safety signals detected.” Conclusion The introduction of durvalumab marks a significant advancement in the management of both BTC and uHCC.5,10 The TOPAZ-1 study established durvalumab combined with Gem-Cis as the first IO treatment and a new SoC for advanced BTC.1,4 The 4-year follow-up of the HIMALAYA study and data from the local subpopulation highlighted the lasting efficacy of the first IO-IO combination.7-9,11 Insights from Dr. He and Prof. Chan on the latest research place the spotlight on these developments and the cases shared by Dr. Chan and Dr. Chiang showed that these results translate into real-world cases. These innovative therapies are transforming the treatment landscape of BTC and uHCC, offering new hope for patients.

