MEETING HIGHLIGHT
Harnessing the potential of deucravacitinib in the treatment of psoriasis: A case series of real-world clinical applications
Psoriasis (PsO) is a chronic immune-mediated inflammatory skin disease characterized by impaired keratinocyte proliferation and differentiation.1 Deucravacitinib is a first-in-class tyrosine kinase 2 (TYK2) inhibitor approved for the treatment of moderate-to-severe PsO.2,3 At a recent symposium chaired by Professor Chan, Hin-Lee Henry, Professor April Armstrong presented the 4-year efficacy and safety data of deucravacitinib from the long-term extension (LTE) of the POETYK trial programs which underscored the efficacy of deucravacitinib in difficult-to-treat areas such as the scalp. In addition, Dr. Guan, Xin and Dr. Zhang, Zhen-Ying also shared their firsthand clinical experience in managing different PsO patient subgroups with deucravacitinib.
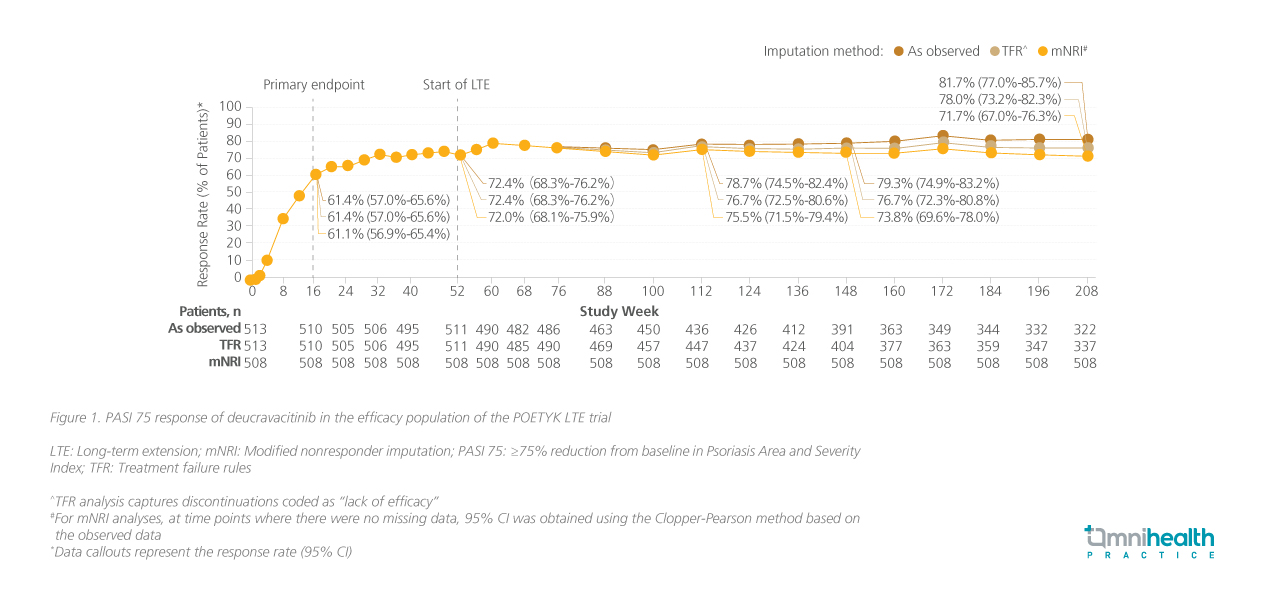
Deucravacitinib demonstrates robust response against moderate to severe PsO sustained at 4 years
Deucravacitinib is a novel oral TYK2 inhibitor approved for treating moderate-to-severe plaque PsO.2,3 It is a selective inhibitor of TYK2 that binds on the allosteric site of TYK2 instead of the catalytic domain.4
In the phase 3 randomized POETYK PSO-1 trial, patients with moderate-to-severe PsO (defined as static Physician's Global Assessment [sPGA] ≥3, Psoriasis Area and Severity Index [PASI] ≥12, and body surface area [BSA] involvement ≥10% for ≥6 months before screening) were randomized to receive deucravacitinib 6mg (n=332), apremilast (n=166) or placebo (n=168) for 52 weeks.4 Compared with apremilast and placebo, deucravacitinib led to a significantly higher rate of ≥75% reduction from baseline in PASI (PASI 75), ≥90% reduction from baseline in PASI (PASI 90), and sPGA of 0 or 1 (sPGA 0/1) at week 16 (p<0.0001 for all except p=0.0002 against apremilast in PASI 90), establishing its potential in minimizing disease activity and symptom improvement.4
Longer-term use of deucravacitinib 6mg daily was investigated in the POETYK open-label LTE.5 Its efficacy was maintained at 4 years with PASI 75 retained throughout the LTE trial (figure 1).5 Prof. Armstrong commented, “I think it is quite notable and uncommon for an oral therapy to maintain such durable responses, which can be attributed to deucravacitinib’s mechanism of action which offers greater selectivity compared with other Janus kinase (JAK) inhibitors.“
A safe and convenient oral option best suited for moderate PsO
When assessing a patient’s eligibility for deucravacitinib treatment, Prof. Armstrong highlighted the importance of balancing disease severity and patient preferences, commenting that “in her clinical experience, deucravacitinib is probably best suited for patients at the ‘moderate’ end of moderate-to-severe PsO.” She emphasized the safety profile, monitoring requirements, potential drug-to-drug interactions (DDIs), and overall patient convenience are the key factors in treatment selection.
Deucravacitinib was well-tolerated among patients with PsO in POETYK PSO-1 at week 16, with a comparable, but numerically lower incidence of serious adverse events (2.1% vs. 5.5%) and discontinuation rate (1.8% vs. 4.2%) compared to the placebo group.4 The most common adverse events (AEs) associated with deucravacitinib were nasopharyngitis, upper respiratory tract infections, and headache.4 The safety profile remained consistent in the pooled safety data of the POETYK PSO-1 and PSO-2 trials in the 4-year LTE.5
Before initiation, only tuberculosis (TB) evaluation was recommended.2 Prof. Armstrong pointed out that liver function tests (LFTs) are recommended before initiation and during treatment, but only in patients with known or suspected liver diseases.2 Additionally, she pointed out that no clinically relevant DDIs have been identified with deucravacitinib, as such monitoring practices are less demanding.2 Given that convenience is an important factor to consider during treatment selection, she also suggested that most systemic treatment-naïve patients may prefer deucravacitinib over biologics due to their familiarity with oral medications and less demanding storage requirements.
Addressing the challenges in managing difficult-totreat PsO
In the pooled analyses of the POETYK PSO-1 and PSO-2 trials, deucravacitinib was also shown to be effective against moderate-to-severe PsO in difficult-to-treat areas defined by a score of ≥3 on the scalp-specific Physician's Global Assessment (ss-PGA), PGA-Fingernails (PGA-F), and palmoplantar PGA (pp-PGA) scores for scalp, fingernail, and palmoplantar PsO respectively.6 The analysis found that deucravacitinib consistently relieved symptoms in these specific areas.6,7 At week 16, patients receiving deucravacitinib demonstrated significantly higher response rates in achieving ss-PGA 0/1 for the scalp (70.3% vs. 17.4%; p<0.0001), PGA-F 0/1 for the fingernails (20.9% vs. 8.8%), and pp-PGA 0/1 for palmoplantar areas (49.1% vs. 16.0%; p<0.0001) compared to those on placebo.6,7 The response rates for all 3 assessments continued to increase after patients in the placebo arm crossed over to deucravacitinib treatment following week 16.7
Deucravacitinib in action: Insights from PsO case experiences
Dr. Guan and Dr. Zhang shared several successful case studies to illustrate deucravacitinib's real-world efficacy in treating diverse PsO presentations. Dr. Guan has treated 49 patients with deucravacitinib so far, leading to effective results in 90% of patients – those with moderate disease responded within 2-4 weeks, while improvements were seen in severe cases within 4-8 weeks. Meanwhile, Dr. Zhang added that the use of biologics was common in her practice. She reported a positive overall impression of deucravacitinib and acknowledged the potential wide application based on the drug’s mechanism and the pathogenesis of PsO. Here are the 2 key cases presented by each speaker at the symposium.
Case #1: Moderate PsO patient with a strong preference against injectable treatments – Dr. Guan
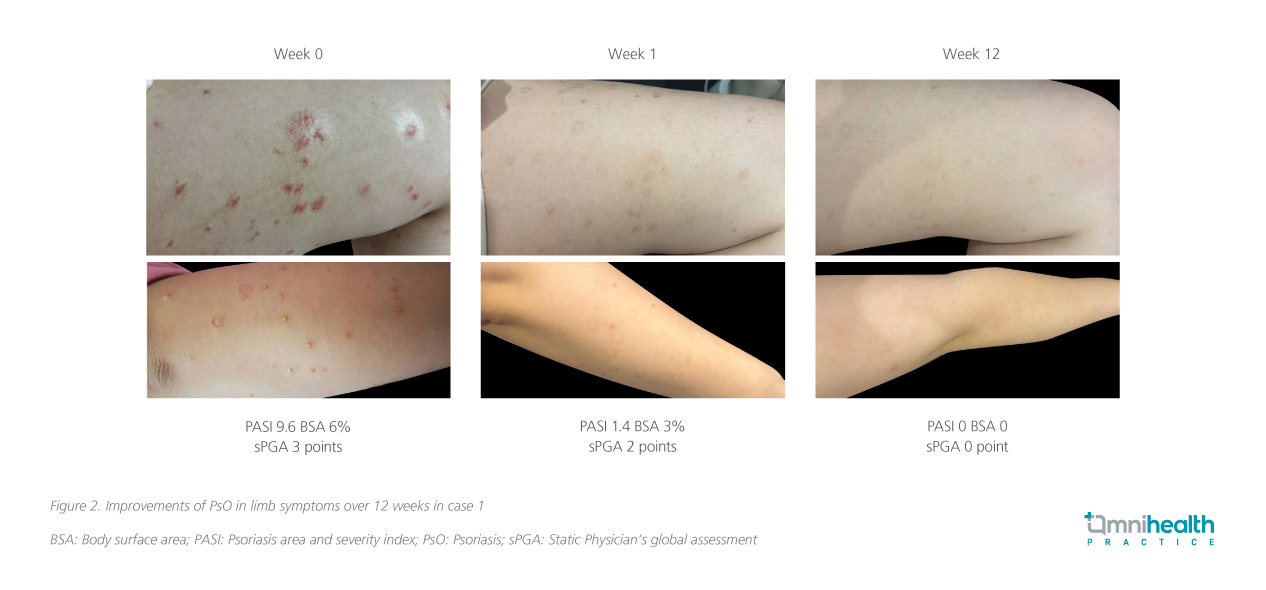
A 21-year-old woman with 4-year history of moderate (6% BSA) PsO, featuring plaques and scaling on torso, limbs and scalp, scored PASI 9.6 and ss-PGA 3 pre-treatment. Despite the ineffectiveness of prior topical and traditional Chinese medicine, she hesitated on the use of injections and was more inclined to receive oral medications in the discussion of treatment options. Deucravacitinib was initiated which led to complete clearance (PASI 0, BSA 0%, ss-PGA 0) after 12 weeks of treatment (figure 2).
Case #2: Moderate PsO patient with scalp involvement – Dr. Guan
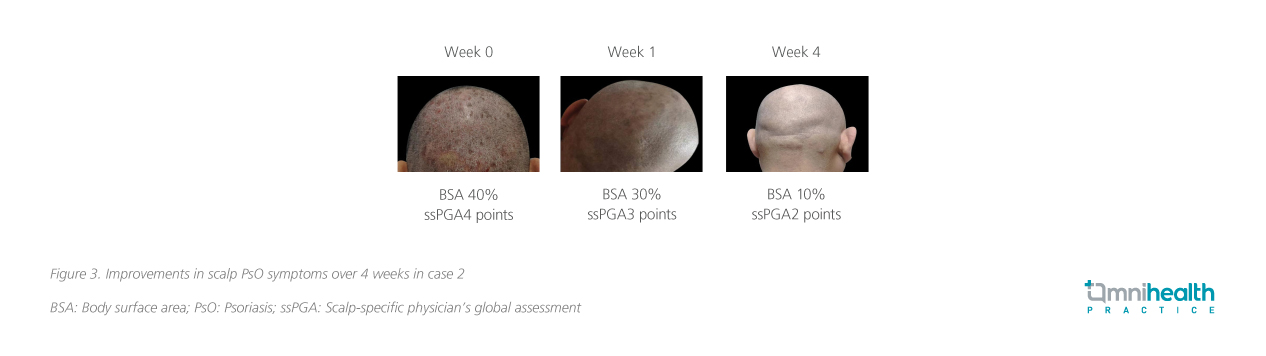
A 52-year-old man with a history of PsO of over 20 years presented with red patches of varying sizes throughout the body. He had received topical treatments such as vitamin D3 derivatives, corticosteroids and Chinese medicinal baths. He had also previously received IL-17A inhibitors such as secukinumab with resistance occurring after 2 years of treatment which led to severe recurrences on both lower limbs, scalp and arms. Before deucravacitinib treatment, he had a BSA of 40%, PASI score 25, sPGA score 5, and a scalp ss-PGA score of 4. BSA improved to 30% at week 1, and 10% at week 4, and an improvement to an ss-PGA score of 2 was achieved by week 4 (figure 3).
Case #3: Moderate PsO patient with progressive small plaques and nail involvement – Dr. Zhang
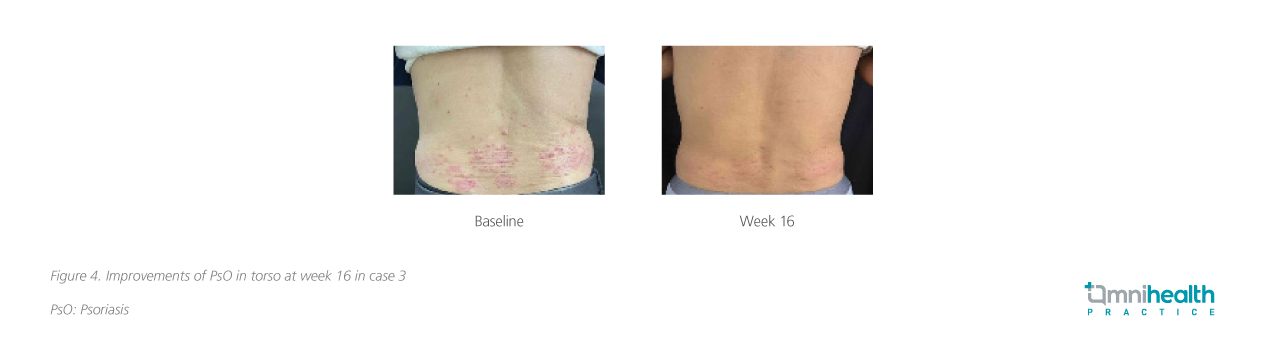
A 74-year-old man with a 10-year history of PsO presented with multiple papules or small plaques over the body (BSA 6%). In addition, nail involvement of PsO was observed, while no joint pain was reported. Topical treatment options such as calcipotriol, betamethasone and fluticasone propionate were offered to this patient, yet yielded suboptimal outcomes, resulting in an elevated frequency of relapse. As no abnormalities were found in blood routine examination, liver and kidney function tests, and hepatitis B and tuberculosis infection indicators, deucravacitinib was administered to the patient, in which PASI 90 was achieved after 16 weeks of treatment (figure 4).
Case #4: A patient with severe, large plaque psoriasis and paradoxical reaction – Dr. Zhang
A 55-year-old man with a 40-year history of PsO was previously treated with secukinumab. However, immune drift led to widespread erythema and exudation. After upadacitinib treatment for eczematous dermatitis, he experienced a paradoxical reaction, triggering a PsO flare and developed severe inflammation (18% BSA). He was also resistant to phototherapy and wet compression, but after starting deucravacitinib, he achieved a PASI 75 by week 8.
Expert perspectives on deucravacitinib in the PsO landscape
Dr. Zhang and Dr. Guan expressed enthusiasm for the potential of deucravacitinib in PsO treatment. They highlighted the medication's unique mechanism as a selective TYK2 inhibitor, which may contribute to an improved safety profile. Crucially, the experts emphasized deucravacitinib's convenience as an oral medication, highlighting its potential to improve adherence compared to injectables. Dr. Guan also recommended incorporating the drug into standard PsO treatment protocols. Overall, the experts expressed strong optimism about deucravacitinib's role in managing moderate PsO.
Conclusion
In conclusion, deucravacitinib is a first-in-class oral TYK2 inhibitor that facilitates effective and durable responses in treating PsO, as demonstrated in the POETYK PSO-1 and follow-up analyses.2-7 Prof. Armstrong highlighted its favorable safety profile, minimal monitoring requirements, and lack of significant drug-drug interactions as key factors that may enhance patient convenience and make deucravacitinib an attractive treatment option, particularly for treatment-naïve patients who may prefer an oral medication over injectable biologics. Real-world experience shared by Dr. Guan and Dr. Zhang further supports deucravacitinib's effectiveness in managing both general PsO and difficult-to-treat scalp manifestations of the condition. With promising results from clinical trials and real-world cases, deucravacitinib emerges as a reliable and valuable treatment option, particularly for individuals with moderate-level PsO.

