EXPERT INSIGHT
Evaluation of cancer risks in IIM: A Hong Kong perspective on IMACS stratification
Idiopathic inflammatory myopathies (IIM) is a group of autoimmune disorders characterized by chronic muscle inflammation, presenting with varying clinical features, treatment responses, and prognoses.1 Recently, the International Myositis Assessment and Clinical Studies Group (IMACS) released a set of international guidelines that offer a foundational overview of cancer risk stratification in IIM, along with corresponding screening strategies for each risk category.2 In an interview with Omnihealth Practice, Dr. So, Ho and Dr. Tang, Yan-Ki Iris discussed their recently published study, which evaluated the applicability and reliability of the IMACS guidelines within the local population of Hong Kong.3 They provided insights into the prevalence of IIM-related cancer in Hong Kong and shared a refined model on how these recommendations can be effectively implemented in the region.
Understanding cancer prevalence in IIM populations
IIM is a group of rare connective tissue disorders with a prevalence estimated at up to 33.8 cases per 100,000 persons.4 Although the link between cancer and myositis was established over a century ago, the underlying mechanisms are not fully understood.4 A diagnosis of cancer-associated myositis (CAM) is made when a malignancy is identified within three years, before or following IIM diagnosis, with up to 25% of IIM patients developing cancer within this period.2,4 Typically, cancer arises concurrently with myopathy or within the first year of diagnosis, but the elevated risk persists for several years.4 Mortality has been seen to increase by more than 3-fold in patients with IIM-cancer (41.7% vs. 12.2%), emphasizing the need for standardized and timely cancer risk stratification and screening to improve survival.3
The IMACS guidelines for IIM-associated cancer screening
Dr. So stated, “While it is recognized that IIM increases the risk of cancer, no international or local guidelines exist. Practices are inconsistent, with no local standards in place, though there is general consensus on the importance of screening. Current approaches remain largely empirical.” To address this elevated risk, the IMACS has recently released a set of guidelines to stratify patients and offer recommendations on respective cancer screening procedures.2 These recommendations propose a strategy to stratify a patient's cancer risk associated with IIM into standard, moderate, or high, based on the IIM subtype, autoantibody status, and clinical characteristics (figure 1).2 Depending on the risk profile, a “basic” screening panel that includes chest radiography and initial laboratory tests, or an “enhanced” panel with CT scans and tumor markers are recommended.2 The timing and frequency of screenings are also tailored to the patient's risk categories.
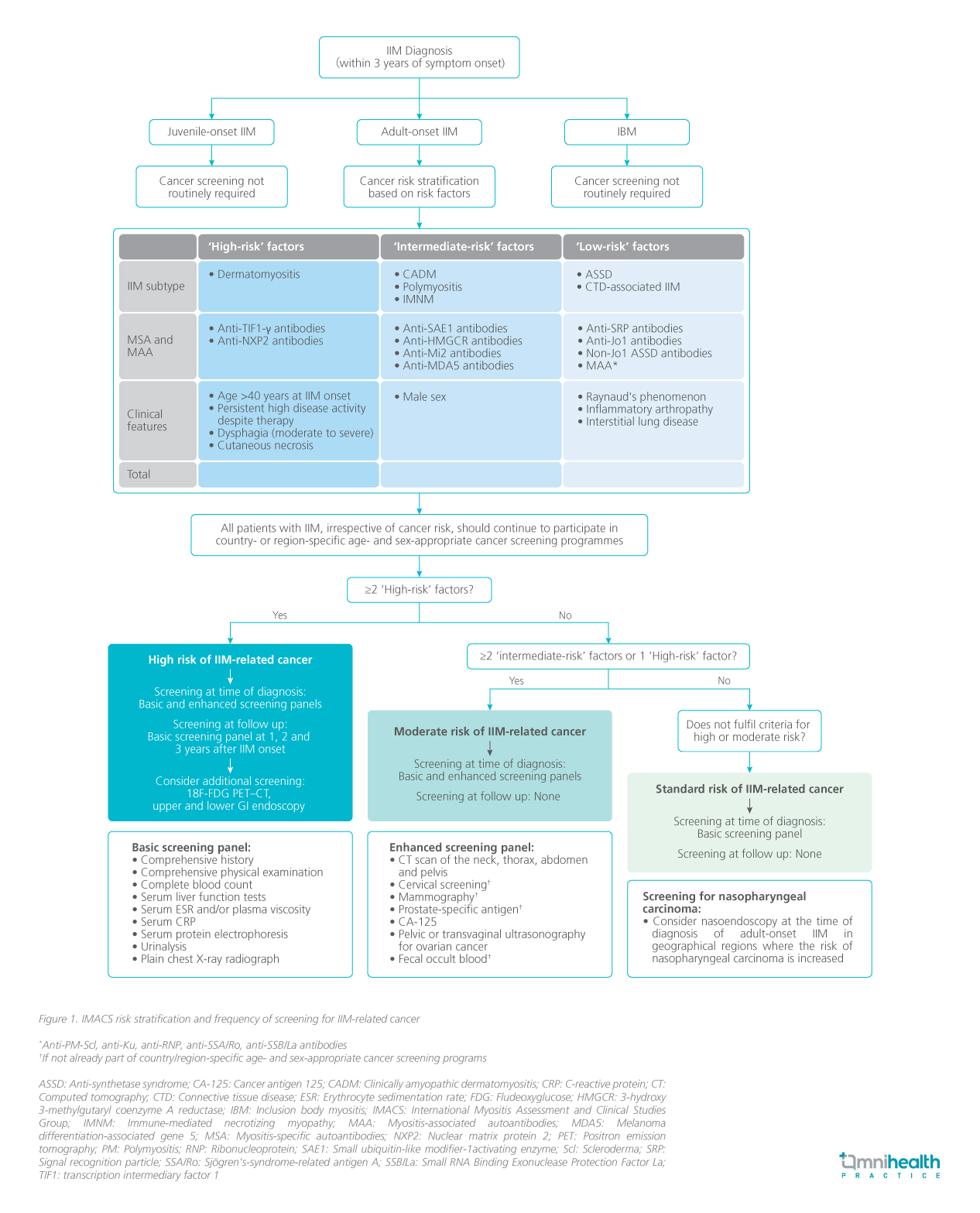
Current frontline screening practices
Dr. Tang shared, “In Hong Kong, patients and physicians are generally aware of the cancer risk associated with IIM, but screening practice is variable. Factors that influence screening are very individualized. For example, the doctor may perform repeated screenings in patients with more active disease or new symptoms, but there is no systematic approach for determining who should be reassessed.” She added that despite high service demand and rising costs due to increased adherence to guideline-recommended screenings, early cancer detection can significantly enhance survival and positively impact costs and quality of life. The guidelines provide a systematic framework for screening and outline the appropriate intensity. However, their external validity had not been verified.3
Bridging the IMACS roadmap of cancer screening in Hong Kong
To evaluate the applicability and reliability of the IMACS guideline locally, Dr. Tang and Dr. So conducted a multi-center retrospective cohort study on its effectiveness in cancer risk stratification using data from a longitudinal IIM cohort across multiple centers in Hong Kong.3 The study aimed to determine the cancer risk profile based on IMACS guidelines and assess cancer detection rates across the different risk categories within the Hong Kong myositis cohort.3 Secondary outcomes focused on identifying risk factors associated with cancer in this population and proposing strategies to improve cancer risk stratification.3
Participants were identified from the Hong Kong Myositis Registry (MyoHK), a territory-wide, population database established in 2019 to collect longitudinal data on local IIM patients.3 Additional patients diagnosed with dermatomyositis, polymyositis, or myositis were included from the Clinical Data Analysis and Reporting System (CDARS), along with patient lists from the 8 participating rheumatology centers.3 Patients aged 18 years or older who met specified criteria for IIM from participating rheumatology centers were included, while those with disease onset before 18 years old, an active malignancy diagnosed within 5 years before their IIM diagnosis, or having unknown myositis-specific antibody (MSA) status were excluded, resulting in 479 patients included in the analysis.3 Electronic patient records from 2004 to 2023 were reviewed, including demographic data, clinical features, and malignancy risk factors according to the IMACS recommendations at the time of IIM diagnosis.3
Cancer screening trends in the local IIM community
The current rates of cancer screening in the IIM population were investigated in the study.3 Between 2018 and 2023, computed tomography (CT) scans of the thorax and abdomen were performed in 53.8% and 21.1% of patients, respectively.3 Additionally, 70.8% of patients underwent positron emission tomography (PET)-CT for cancer screening at the time of their IIM diagnosis.3 Overall, 86.5% received either a CT thorax and abdomen or a PET-CT as part of their cancer screening.3 Nasoendoscopy was conducted in 76.6% of patients, tumor markers were assessed in 83.6%, while abdominal and pelvic ultrasonography were performed in 23.4%.3 Notably, only 5 out of 171 patients did not undergo any of these screening procedures.3
Mapping the risk profiles in local IIM populations
After a brief overview of the screening measures conducted in the IIM population, the study identified key trends in IIM patients stratified by cancer diagnosis.³ Patients with cancer within 3 years were older (62.3±13.3 years) than non-cancer patients (53.4±13.3 years; p<0.001) and more often male (43.3% vs. 30.1%; p=0.039).³ Multivariable analysis indicated that older age (odds ratio [OR]=1.048), Gottron’s rash (OR=2.453), anti-TIF1 positivity (OR=4.627), and anti-SAE1 positivity (OR=5.325) were significant clinical and serological risk factors for cancer.³ Cancer patients were also more likely to present with dermatomyositis (55.0% vs. 24.1%; p<0.001) and heliotrope rash (53.3% vs. 31.5%; p<0.001).³ Additionally, double MSA positivity was higher in cancer patients (16.7% vs. 5.7%; p=0.002).³
Conversely, interstitial lung disease (ILD) was negatively associated with cancer (18.3% vs. 58.2%; p<0.001) and anti-Jo1 positivity was lower in cancer patients (3.3% vs. 17.9%; p=0.004).³ The levels of non-Jo1 anti-synthetase antibodies were similar between groups (21.7% vs. 18.4%; p=0.542), while anti-MDA5 (1.7% vs. 15.8%; p=0.003) and myositis-associated autoantibodies (MAAs) (43.3% vs. 55.6%; p=0.071) were more common in non-cancer patients.³
Based on the IMACS cancer risk stratification at the time of IIM diagnosis, 442 patients (92.3%) had ≥1 high-risk factor.3 In total, 214 patients (44.7%) had ≥2 high-risk factors for cancer and were classified into the high-risk group, 49.7% were classified into the intermediate-risk group, and 5.6% into the standard-risk group.3 Of those in the high-risk group, 20.6% were diagnosed with cancer within three years, compared to 6.7% in the intermediate-risk group and 0% in the standard-risk group (p<0.001).³ Notably, 75% of cancer cases were detected at initial presentation.³ The high-risk group had significantly higher cancer incidence than the intermediate (p<0.001) and standard-risk groups (p=0.009).³ However, there was no significant difference between the intermediate- and standard-risk groups (p=0.165).³ Among the 228 intermediate-risk patients, 95.8% had one high-risk factor, primarily age >40 years (90.8%), with 40.8% showing no additional intermediate risk factors at diagnosis.³
Regarding the stratification in local populations, Dr. So stated “We are very glad for the guideline, but we need to see if local adaptation is needed. In fact, many patients need to receive high-intensity screening. However, the question remained – are there any methods to further refine stratification?”
Charting the course for local adaptation and further investigations
The study revealed that a single high-risk factor and age of over 40 years, accounted for more than 90% of the high-risk factors identified.3 This age threshold of 40 was derived from studies of anti-TIF1γ positive patients, indicating an increased cancer risk beyond this age.5,6 While older age is generally recognized as a cancer risk factor, no specific age cut-off has been established for anti-TIF1γ negative populations.3 It was discussed that the 40-year cut-off seemed appropriate when other high-risk features were present; however, applying this threshold without additional risk factors may result in over-screening.3 Further evaluation of cancer association with different age cut-offs in the intermediate subgroup revealed no cancer cases in patients aged 45 years or younger.3 Consequently, it was proposed that for patients aged 45 years and under in the intermediate group, physicians may consider standard cancer screening akin to the standard risk group unless other symptoms or a family history of cancer are present.3 As such, Dr. So, Dr. Tang, and their team proposed a revised screening flowchart for CAM in Hong Kong (figure 2).3
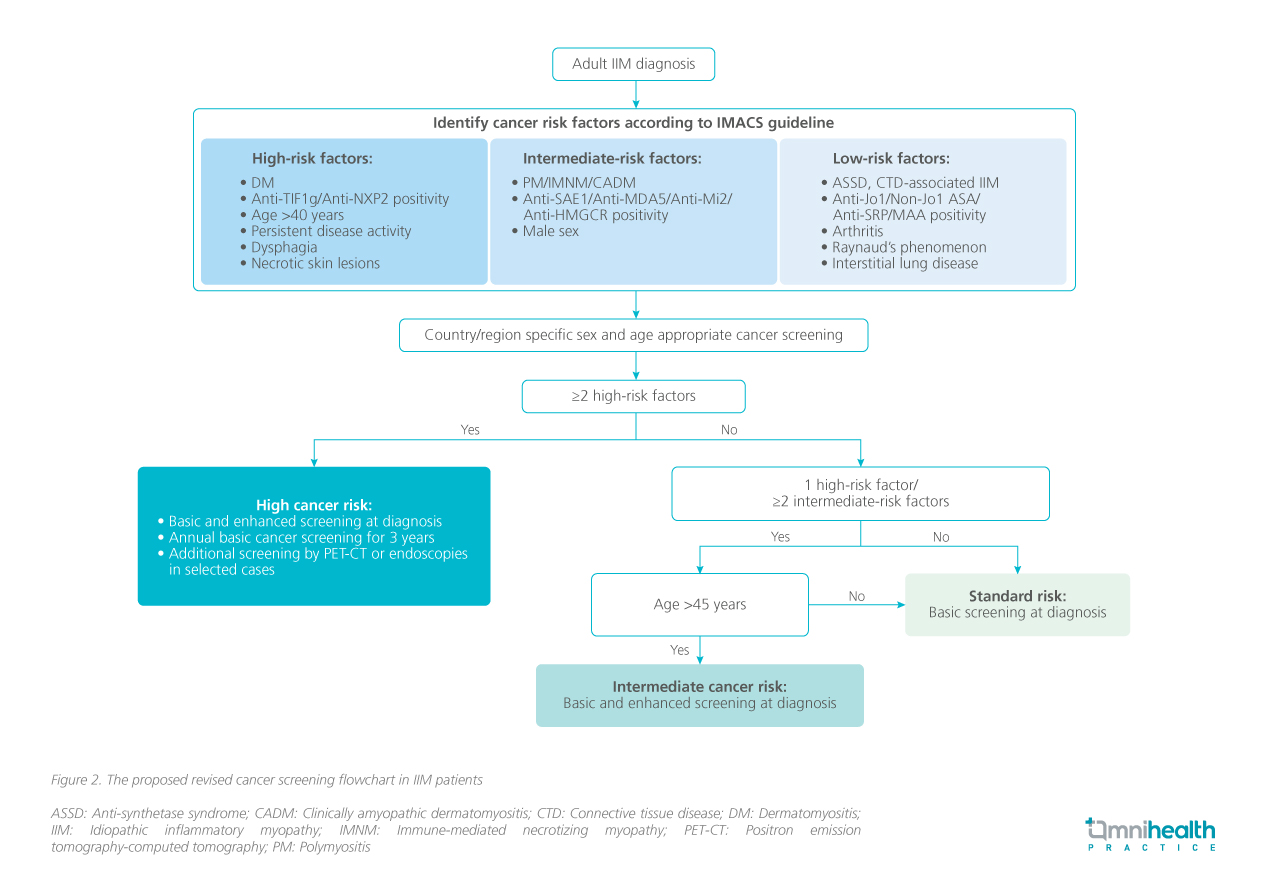
Conclusion
Current cancer screening practices for I IM in Hong Kong are inconsistent. Research by Dr. So, Dr. Tang, and their team suppor ts the accuracy of the cancer risk stratification framework developed by IMACS, demonstrating that most IIM patients would undergo extensive screenings according to these guidelines.3 To prevent service burden, prioritizing enhanced screening for patients over 45 years in the intermediate risk group could be beneficial without compromising cancer detection.3

