FEATURES
Lasting protection from recurrence: A case sharing of high-risk HR+ HER2- EBC management with abemaciclib
Abemaciclib was the first cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor to demonstrate a significant improvement in invasive disease-free survival (IDFS) in patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) early breast cancer (EBC).1 In an interview with Omnihealth Practice, Dr. Hui, Cheng-Vai cited data from large-scale clinical trials including monarchE, describing the long-term efficacy and tolerable safety profile of the 2-year fixed-duration abemaciclib treatment, even in populations who had received dose reductions. Dr. Sumou, Ingrid Karmane also shared her experience using abemaciclib in a case of an HR+ HER2- EBC patient with a high risk of recurrence, outlining the efficacy of the treatment and how its tolerability profile can be managed. Additionally, they highlighted the importance of patient education and close follow-up by a multi-disciplinary team to enhance treatment outcomes.
The call for adjuvant abemaciclib in HR+ HER2- EBC
Most patients (around 90%) with breast cancer are diagnosed in the early stages.1 A large proportion (around 70%) of these patients fall in the HR+ HER2- category.1 Up to 37% of these patients experience disease recurrence within 5 years, often with distant metastases that are incurable.2 Optimizing adjuvant therapies is therefore essential in preventing recurrences and metastasis in these patients.1 Dr. Hui highlighted that the Macau public health administration endorses a motto of “appropriate treatment, prioritize prevention,” recognizing that early intervention may decrease the number of patients developing late-stage disease, reduce recurrence rates, and improve cost-effectiveness.
Abemaciclib is an oral, continuously dosed CDK4/6 inhibitor that has been shown to benefit HR+ HER2- node-positive EBC patients at high risk of recurrence.1 The addition of 2 years of abemaciclib to endocrine therapy (ET) in the adjuvant setting is the standard of care for HR+ HER2- node-positive EBC patients at high risk of recurrence as recommended by both the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) guidelines.3,4 Dr. Hui commented, “Compared with other options, the 2-year treatment duration is quite short.” He further highlighted the overall cost benefits, given the short drug duration and lower overhead costs associated with less frequent follow-up. To illustrate the benefits of early adjuvant therapy with abemaciclib, Dr. Sumou shared a case example from her practice of its use in the management of an HR+ HER2- node-positive EBC patient.
Case sharing
The patient is a 37-year-old premenopausal Chinese woman with no significant medical history or family history of breast/ gynecological cancer. She was diagnosed with right breast cancer, stage IIIc (pT3N3a), after presenting with a 6.7cm tumor with compromised margins, multifocal lymphovascular invasion, high-grade ductal carcinoma in situ (DCIS), and metastatic carcinoma in 13 out of 22 lymph nodes up to 1cm in diameter. The tumor was grade 3 with an estrogen receptor (ER) Allred score of 7 and a progesterone receptor (PR) Allred score of 6. There was a high antigen Kiel 67 (Ki67) proliferation index of 35%, with a HER2 immunohistochemistry score of 1 and negative for amplification by dual in situ hybridization (DISH).
She underwent a mastectomy with breast reconstruction, followed by 8 cycles of anthracycline-based adjuvant chemotherapy (fluorouracil, epirubicin hydrochloride, and cyclophosphamide) and 4 cycles of docetaxel starting in November 2021 and completing by April 2022. She also received goserelin, then later leuprorelin, for 1 year and adjuvant radiotherapy to the right chest wall and lymphatic regions which was completed in August 2022. In November 2022, she started receiving abemaciclib 150mg twice daily, intended for 2 years, along with tamoxifen.
Upon initiating abemaciclib, she experienced grade 2 diarrhea. However, the patient was reluctant to take loperamide to manage diarrhea, prompting her to skip up to two doses per week during the initial 3 months. Over time, her diarrhea improved to grade 1 and the treatment has been well-tolerated overall. Treatment is still well-tolerated and ongoing. No dehydration, electrolytic imbalances, weight change, or renal problems were reported as a result of treatment or diarrhea.
As of April 2024, after 17 months of abemaciclib treatment, the patient's follow-up breast imaging (mammogram and ultrasound) showed benign findings without evidence of malignancy. The patient has confidence in the treatment and is being closely monitored.
MonarchE: 5-year efficacy of abemaciclib after 2 years of treatment
The robust efficacy of abemaciclib observed in this case aligned with the data from the phase 3 monarchE trial.5 The monarchE trial was an open-label, phase 3 study that included patients with HR+, HER2-, node-positive high-risk EBC.1 Eligible patients were divided into 2 cohorts.5 Patients in cohort 1 had ≥4 positive pathologic axillary lymph nodes (pALNs) or 1-3 pALNs with high-risk features of either histologic grade 3 or tumor size ≥5cm.5 They were randomly assigned (1:1) to receive standardof- care (SoC) adjuvant ET with or without 150mg abemaciclib twice daily for 2 years.1,5
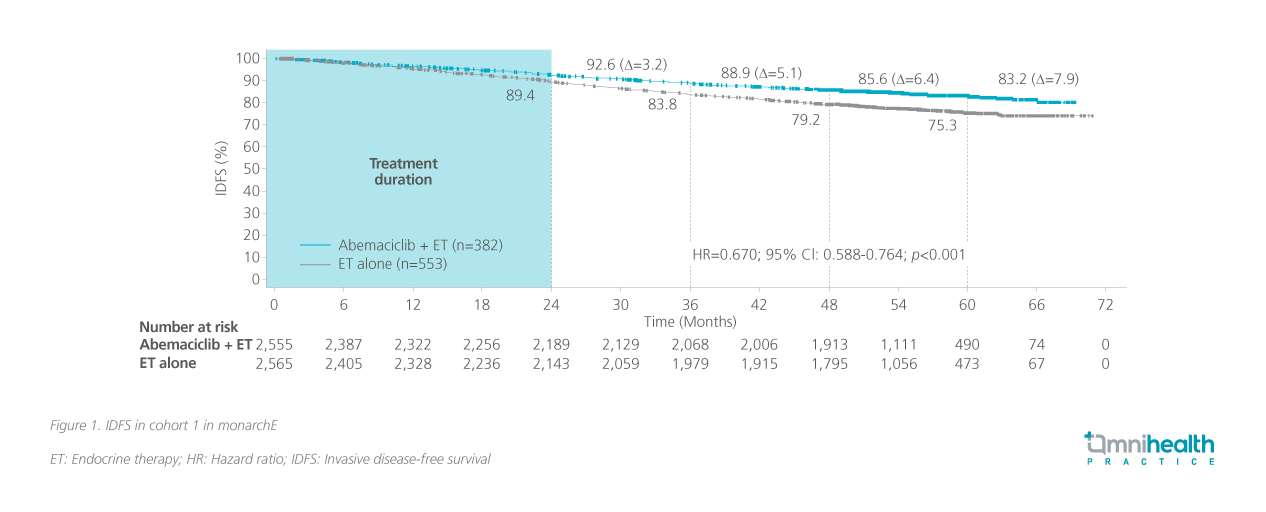
The monarchE trial used IDFS as the primary endpoint.1 Traditional endpoints such as overall survival (OS) require a substantial number of patients and follow-up duration to be effectively evaluated.6 Dr. Hui admitted that evaluating these conventional endpoints requires resources and time, which the current patient population does not have. As such, novel endpoints were established to reflect the priorities of EBC management in this population.6 He cited the Food and Drug Association’s (FDA’s) endorsement of the use of disease-free or event-free survival as clinical or surrogate endpoints for both traditional and accelerated approvals.7 IDFS was measured from the date of randomization to the date of the first occurrence of ipsilateral invasive breast tumor recurrence, local/regional invasive breast cancer recurrence, distant recurrence, death attributable to any cause, contralateral invasive breast cancer, or second primary non-breast invasive cancer as confirmed by biopsy or imaging.1 Secondary endpoints included distant relapse-free survival (DRFS), OS and safety.1 At the latest 5-year follow-up of monarchE, abemaciclib + ET continued to reduce the risk of IDFS beyond the completion of treatment, with an increasing absolute improvement at 5 years, suggesting a carryover effect (figure 1).5 The benefits within cohort 1 were also maintained in DRFS (HR=0.665; 95% CI: 0.577-0.765; p<0.001).5 OS results were still immature, but a survival signal favoring abemaciclib is emerging.5
Enhancing tolerability with dose adjustments
The patient in the case experienced mild tolerability issues in the form of diarrhea, which was consistent with the tolerability profile observed in the monarchE trial population.8 Dr. Sumou noted that diarrhea in this patient was mostly resolved after the third month and did not require substantial dose reduction. In a safety analysis of monarchE, discontinuation of study treatment due to adverse events (AEs) occurred in 18.5% of patients.8 Diarrhea was generally low grade with the majority (76%) being grade 1/2.8 Grade 2 or 3 events were the most common in the first month of treatment at 20.5%.8 It was reported that after the initial 6 months, diarrhea was generally grade 1 and was intermittent.8 However, most of these were reported to be short-lived (<7 days) and did not recur.8
In monarchE, dose reductions were allowed to proactively manage AEs.9 AEs were mainly managed with over-the-counter comedications (e.g. antidiarrheals), abemaciclib dose holds (61.7%), and/or dose reductions (43.4%).8 Diarrhea was the most common AE cited as the reason for dose holds (19.5%) and dose reductions (17.3%).8 An exploratory analysis of the impact of dose reductions in these populations revealed that protocol-mandated dose reductions did not compromise the efficacy of abemaciclib treatment.9 Across the 3 patient subgroups which received a relative dose intensity of ≤66%, 66%-93%, and ≥93% respectively, the estimated 4-year IDFS rates were similar at 87.1%, 86.4% and 83.7%, respectively in the ITT population (figure 2).9 These results were generally consistent in cohort 1 at 87.2%, 86.1% and 83.1% respectively.10 Dose adjustments may be offered to patients receiving adjuvant abemaciclib to maximize adherence and maintain benefit from therapy.10

The role of patient education in treatment adherence
Dr. Hui and Dr. Sumou emphasized the significance of maintaining treatment adherence during abemaciclib adjuvant therapy. Dr. Sumou specifically highlighted the role of frequent follow-up and a strong patient-provider relationship in achieving treatment success. Given that the effects of treatment in EBC may not be immediately apparent compared to its AEs, patient education, and vigilant monitoring play crucial roles in optimizing outcomes. Treatment duration and potential AEs should be communicated, and physicians should help instill greater confidence in patients about their prescribed therapy.
In Dr. Hui’s experience, the initial 3 months of treatment have the highest dropout rate, but patients’ care journey tends to stabilize afterward. A multidisciplinary approach is essential to overcome any challenges in treatment to provide stability throughout the journey. Additionally, an early, proactive, and customized approach should be adopted to manage diarrhea in clinical practice.8 Patients should be advised to be vigilant for the onset of diarrhea and promptly start antidiarrheal medication at the first sign of loose stools.8
Conclusion
The landmark monarchE trial demonstrated the long-term benefits of adjuvant abemaciclib treatment for patients with HR+ HER2- EBC at a high risk of recurrence.5 Despite the short duration of treatment, IDFS benefits are sustained through a 5-year followup with a manageable AE profile.1,5 In the outlined case, the early introduction of abemaciclib prevented relapse of EBC while maintaining a manageable tolerability profile, especially after the initial 3 months. Dr. Hui and Dr. Sumou lastly emphasized the critical role of patient education in promoting treatment adherence, ensuring that patients derive the maximum benefit from their treatment.

